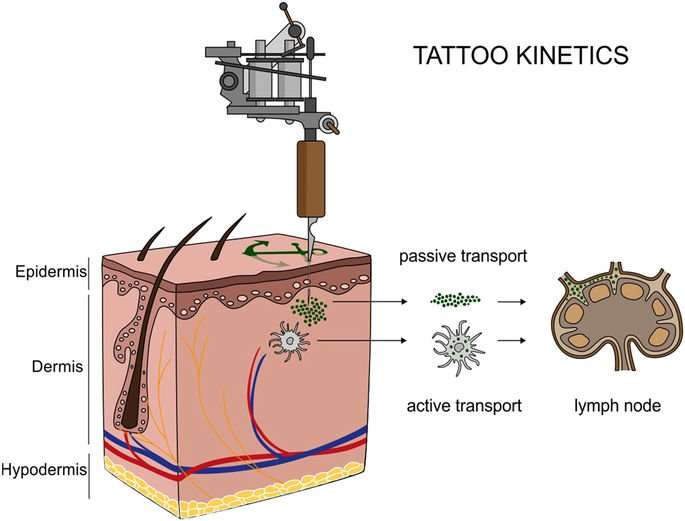
Organic pigments translocate from skin to lymph nodes
Providing analytical evidence of tattoo particles being distributed inside the human body was a key objective of this investigation. To this end, tissue samples of four individuals tattooed with orange, red, green or black and two non-tattooed control donors were analyzed for the presence of organic pigments. Detection of the same pigment species in both skin and regional lymph nodes revealed the drainage of tattoo particles in two out of four tattooed donors (Fig. 2).
Figure 2 Organic pigments translocate from skin to lymph nodes. Organic pigments in lysed skin and lymph nodes were identified by means of LDI-ToF-MS. Adjacent skin and lymph tissue specimens (about 5–10 mm) are displayed in cryo-matrix after preparing thin sections for μ-FTIR and μ-XRF analyses. Skin specimens are oriented with its surface on the right side. Identified organic pigments are indicated below each sample. Chemical structures of the organic pigments identified in the samples are displayed on the right. Full size image
Identification of organic pigments using LDI-ToF-MS has mostly been described using inks21,22,23. This technique is mainly based on isotope distributions and the molecular mass (see Supplementary Fig. S1). In the lysed tissues presented here, color-giving pigments were found to be copper phthalocyanines with either hydrogen, chlorine or bromine residues in three out of four skin samples. Reddish parts of the tattoos contained the azo group-containing pigments red 170 and orange 13 (Fig. 2).
For donors 1 and 2, the absence of organic pigments in the lymph nodes suggests either concentrations below the limit of detection (approx. 0.1–1% w/w pigment per extract), possible metabolic decomposition or drainage to alternative lymph nodes. The general ability for azo pigment translocation to lymph nodes was proven in additional skin and lymph node samples of donor 2 (Supplementary Table 1). On the other hand, carbon black particles possibly responsible for the black color in skin and lymph nodes (Fig. 2) were not accessible with the analytical methods used in this investigation. No xenobiotic pigment particles were detected in either skin or lymph tissue of the control samples.
Tattoos contribute to the elemental load of lymph nodes
A central aim of this study was to assess to what extent tattooing increases the proportion of toxic elements in the body. We found Al, Cr, Fe, Ni and Cu quantitatively elevated in skin and lymph node specimens using ICP-MS analysis (Table 1 and Supplementary Table S2). For donor 4, Cd and Hg concentrations were found increased only in the lymph nodes, but not in the analyzed skin sections. These elements probably result from other tattoos that were not part of this study or other routes of exposure drained through the same lymphatic tissue. Non-quantitative evaluation of the survey scans revealed the presence of Ti, presumably derived from TiO 2 , in all tattooed skin samples but not in controls.
Table 1: Element concentrations per tissue weight (ppm) in human skin and lymph node samples analyzed by ICP-MS. Full size table
The microwave digestion used in this investigation is not suitable to fully dissolve Fe and Ti, although no residual particles were visible. Therefore Fe concentrations might not represent the total amount in the samples, but they enable the distinction between physiological concentrations in controls and samples containing extrinsic Fe. The elevated levels of Fe found in the skin and lymph nodes of donor 4 imply an additional use of iron-based pigments. In donors 1, 2 and 3, Fe concentrations were only increased in adjacent lymph nodes and not in the corresponding skin samples (Table 1). Fe concentrations can also be affected by residual blood within the tissue samples.
In donor 4, the use of pigment copper phthalocyanine green 36, as identified with LDI-ToF-MS, is reflected by high amounts of Cu in skin and lymph nodes as well as the non-quantitative detection of Br (Table 1). By contrast, although pigment copper phthalocyanine green 7 was well detectable with our LDI-ToF-MS approach in the skin of donor 2, it was not in the corresponding regional lymph node. Increased Cu levels measured by ICP-MS in this adjacent sample, however, suggest the presence of this copper phthalocyanine pigment. In light of the other two copper phthalocyanines applied in donor 2 (green 7) and 3 (blue 15) elevated Cu levels in skin came without surprise (Table 1). In donor 2, Cu levels in lymph nodes are strongly increased despite the fact that green 7 could not be detected with LDI-ToF-MS. However, adjacent samples of tissue were used for each analysis. Given the nature of the samples, pigment deposition within skin and lymph nodes is not homogeneous and therefore explaining the different findings. Interestingly, the non-tattooed control donor 1 also had slightly elevated levels of about 13 ppm Cu in the lymph nodes which is still in the range of the average 5.89 ± 8.03 ppm of Cu detectable in lymph nodes of female cadavers (Table 1)24.
Additionally, Ni and Cr were found in the human specimens. Since Ni levels were increased in the skin and lymph nodes of donor 2 and 3, the likely source is the tattoo. In different studies, both elements were linked to adverse reactions occurring in tattooed patients25,26,27,28. Ni and Cr are known to be allergenic as well as carcinogenic. Ni concentrations of 0.28–10.05 ppm total tissue weight found here are within the range of 0.8–3.7 ppm dry weight Ni in hilar lymph nodes in previous studies29. Cd was drastically elevated only in the lymph node of donor 4. For all other samples, Cd tissue burdens lie within normal values24.
Finally, Al was also present in skin and lymph node tissues of the three tattooed donors 2, 3 and 4 (Table 1). Since auxiliary lymph nodes have been investigated in the case of donor 2 and 3, co-exposure from antiperspirants containing various aluminum salts cannot be excluded, neither in tattooed nor control samples. However, Al concentrations in the controls were lower. The light metal Al has recently attracted attention because of its accumulation in breast cancer tissue30. While its role in the emerging of neoplasia is currently highly disputed, its contribution to the occurrence of hypersensitivity granulomas associated with tattoos has been proven since decades31.
μ-XRF mapping links metallic elements to tattoo particles
In order to link elements found with ICP-MS in tattoo pigment particles and to locate them inside the tissues, μ-XRF imaging was carried out with sub-micrometric probes over skin and lymph node sections (Fig. 3a–d). The location of particles can be altered by sample preparation. Since transversal sections were made by moving the knife parallel to the skin surface, the depth profile of the pigments should remain unaffected. Thin sections of skin and lymph nodes from donors 1, 3 and 4 were analyzed at the ESRF beamline ID21, with an exciting energy of 5.05 keV (Fig. 3a–d and Supplementary Fig. S2). Since the thin sections were deposited on BaF 2 windows for further μ-FTIR analyses, the energy was chosen to avoid excitation of Ba L-lines (<5.24 keV). Results of donor 4 are displayed in Fig. 3 as an example.
Figure 3 μ-XRF mapping identifies and locates tattoo particle elements in skin and lymph node tissue sections. Sections of skin and lymph node tissue from donor 4 were analyzed by means of synchrotron μ-XRF. (a) Visible light microscopy (VLM) images of the area mapped by μ-XRF. Tattoo pigments are indicated by a red arrow. (b) DAPI staining of adjacent sections showing the cell nuclei. (c) μ-XRF maps of P, Ti, Cl and/or Br. For the lymph node, areas of similar size are marked in (a) and (b). (d) Average μ-XRF spectra over the full area displayed in (c) *diffraction peak from sample support; **scatter peak of the incoming beam. (e) Ti K-edge μ-XANES spectra of skin and lymph node compared to transmission XANES spectra of reference material of rutile, anatase and an 80/20 rutile/anatase mixture calculation. Full size image
The majority of particles in the skin tissue were surrounded by phosphor-rich nuclei visualized by DAPI staining in fluorescence light microscopy (Fig. 3b) and integration of the element P in μ-XRF analysis (Fig. 3c). It was previously shown that tattoo particles can primarily be found around vessels10 which might account for the high cell density in the dermis co-localized with the pigments.
Intensities of Ti K-lines and Br L-lines were extracted to map the distribution of TiO 2 and the highly brominated pigment copper phthalocyanine green 36 (Fig. 3c). Since the Br L-lines completely overlap with the Al K-lines, both may contribute to the intensity of the peak. However, LDI-ToF-MS analysis revealed the presence of pigment green 36 (Fig. 2) and the following ν-XRF results from ID16B acquired at 17.5 keV, i.e. above Br K-edge (13.47 keV) undermined the primarily Br-related contribution (see Supplementary Fig. S3).
Tattoo particles containing Ti and Br are adjacent to each other with only a slight overlap in skin and seem to be more evenly co-localized in lymph tissue (Fig. 3c). Both elements were found in the dermis of donor 4 directly beneath the cell nuclei-rich epidermis and up to a few hundred micrometers deep in the skin. In the lymph nodes, some particles were deposited in the stroma directly beneath the capsule. The bulk of Ti and Br containing particles, however, became visible as pigment agglomerates at a distance of about 250 µm to the lymph node capsule. Conversely, Cl concentrations are highest in the lymph node capsule and lower concentrations can be found in the particle region as part of the pigment phthalocyanine green 36.
All analyzed samples from the tattooed donors contained Ti. It is unlikely that other sources, e.g. sun screens, would explain the high amounts found in this investigation. Elevated amounts of Ti are only expected in lung and hilar lymph nodes from respiratory exposure32. Other highly abundant elements are K and Ca as they are physiologically present in cells (Fig. 3d).
We also investigated if the Ti present is the expected white pigment TiO 2 and whether the stable rutile and/or the more photoreactive anatase crystal phases were used in tattoo inks. Micro X-ray absorption near edge structure (μ-XANES) spectra at the Ti K-edge were collected for the skin and lymph nodes of donors 1, 3 and 4. The spectra of donor 4 showed more qualitative correlation with the reference spectrum of rutile than with that of anatase (Fig. 3e). A clear switch of peak maxima between 4.99–5 keV occurs as a difference of both types of crystal structures. A calculated spectrum of 20% anatase and 80% rutile mixture is not clearly distinguishable from pure rutile, but shows a pre-edge at around 4.97 keV, similar to the μ-XANES spectra of the tattooed samples. Therefore, mostly rutile TiO 2 is present in all tattooed donors, with minor amounts of anatase (Fig. 3e and Supplementary Fig. S2).
Particle size varies between pigment species
The obtained μ-XRF maps of skin and lymph node sections show large tattoo particle agglomerates up to several micrometers (Fig. 3c). However, it is known that small-sized particles are preferentially transported to lymph nodes. The 0.3 × 0.7 µm² beam size for μ-XRF mapping at ID21 was therefore a limiting factor for the determination of particle sizes. To assess the primary particle sizes, we additionally performed ν-XRF investigations by applying a beam of 50 × 50 nm² at 17.5 keV in order to excite the Br K-lines. Experiments were carried out in adjacent sections of skin and lymph node from donor 4, prepared on ultralene foil (Fig. 4). We detected three different pigment particles, each showing a different elemental composition and distribution within the same area (Fig. 4b,e). The average particle size of TiO 2 in both skin and lymph nodes was 180 nm with a standard deviation of 23 nm and a standard error of 7 nm. Therefore this rather large particle size does not prevent distribution via the lymph fluid.
Figure 4 Particle mapping and size distribution of different tattoo pigment elements. Skin and lymph node of donor 4 were analyzed by means of synchrotron ν-XRF. (a,d) Ti and the Br containing pigment phthalocyanine green 36 are located next to each other. Average XRF spectra over the full area displayed in the regions of interest reveal the presence of Br, Si, S, Cl, Ca, Ti, Cr, Fe, Ni, Cu, and Zn. (b,e) Log scale mappings of Ti, Br and Fe in the same areas as displayed in (a) and (d) reveal primary particle sizes of different pigment species. (c,f) Magnifications of the indicated areas in (b) and (e), respectively. Full size image
In contrast, the pigment phthalocyanine green 36 analyzed by ν-XRF mapping of Br was much more polydisperse, with particles presumably smaller than the resolution of 50 nm and up to the µm range in skin. In lymph node tissue, particles containing Br were smaller, with fewer particles of a larger size (Fig. 4c,f). Hence it can be assumed that the transport of smaller particles is preferential.
With the chosen energy, Br can be unequivocally identified from its K-lines emission. The skin and lymph node of donor 4 also contained Cu, related to the identified copper phthalocyanine pigments, and its maps show perfect co-localization with Br (see Supplementary Fig. S3). Additionally, Fe particles were present in the lymph node but not skin tissue and therefore possibly originate from another tattoo or route of exposure (Fig. 4c,f and Supplementary Fig. S3).
The synchrotron-based μ-FTIR end-station at ID21 was used to monitor changes in protein conformation as well as in the overall protein and lipid contents in the proximity of tattoo particles. Synchrotron μ-FTIR analyses allow the assumption that tattoo pigments became located in a lipid-rich β-sheet protein environment.
The very same sections investigated by means of μ-XRF at ID21 were analyzed by means of μ-FTIR, prior to X-ray analyses, to facilitate exact site matching (cf. Figures 3 and 5). Thus, μ-FTIR results were not altered by μ-XRF radiation of the tissue sections. The high synchrotron photon flux allowed for high spatial resolution. Accordingly, the beam and pixel sizes were reduced to 10 × 10 µm² and 8 × 8 µm², respectively. This resolution is sufficient to distinguish regular dermis from pigment containing areas in the dermis, but remains insufficient to unambiguously separate the stratum corneum from the epidermis, which were analyzed here as a single domain (see below). Specific spectral changes related to the modification of biomolecule composition and conformation are displayed using donor 4 as an example, on the basis of two μ-FTIR maps obtained in a single section at two different locations, for the skin and regional lymph node (Fig. 5). The absorption band which peaks at 2920 cm−1 corresponds to the –CH 2 stretching mode, which is much more intense in lipids than in proteins. It can be used to qualitatively map the distribution of lipids over thin sections (Fig. 5a). It shows a higher intensity in the stratum corneum, as expected33. These maps also qualitatively show a higher intensity in the areas of dermis containing tattoo pigments compared to pigment-free control regions. Based on the microscopic images and the μ-XRF maps described earlier, three regions were selected on each map. For the skin section, we divided the obtained map into stratum corneum and epidermis (SC), dermis without pigment (D) and dermis around pigment particles (DP) (Fig. 5a). Spectra in the second derivative of these areas were statistically analyzed by means of Principal Component Analysis (PCA). Distribution of points along the PC-1 axis confirms that D and DP have fewer lipid-related long alkyl chains (–CH 2 stretching mode, asym. at 2920 cm−1 and –CH 2 sym. at 2854 cm−1) and ester (–C = O stretching mode, peak at 1745 cm−1) vibrations than SC, and that DP regions contain higher levels of lipids than D (Fig. 5b–d). PC-2 separates DP from D and SC since the latter two have higher protein concentrations.
Figure 5 Changes of the biological composition and structure in the cellular proximity of tattoo pigment particles. Section of donor 4 analyzed by means of synchrotron μ-FTIR at ID21, ESRF. (a,e) Maps in second derivative obtained at 2920 cm−1 (–CH 2 asymmetric vibration) of two different areas in either the skin or lymph node of donor 4 in overlay with a visible light microscopy image. Single points for PCA analysis in (c) and (g) were picked from the indicated areas. (b,f) Mean spectra from each region marked in (a,e) in second derivative. (c,g) PCA score plot of PC-1 vs. PC-2. (d,h) Loading plots of PC-1 and PC-2. Abbreviations: SC = stratum corneum and epidermis; D = dermis; DP = dermis with particles; P1, P2 = particle-containing regions; C1, C2 = control regions without particles. Full size image
kungfoojesus on September 13rd, 2017 at 00:10 UTC »
As someone who reads lymphoscintigraphy and sees all manner of foreign objects, especially breast implants, leak and end up in lumpy nodes, this is completely unsurprising. That's where shit goes, I'm not sure what was expected
gnomes616 on September 12nd, 2017 at 22:44 UTC »
I have quite a few tattoos, and one of the pathologists I worked with said there was a correlation between tattoos and getting lymphoma later on. Many searches proved this untrue, but a different pathologist said it can make it more difficult to detect metastatic melanoma in lymph nodes (especially with dark ink).
Campmasta on September 12nd, 2017 at 20:39 UTC »
ELI5? I'm still working on my half sleeve tattoo and wondering if I now have cancer..... Thanks!