In 1985, atmospheric scientists in Antarctica noticed something troubling. For decades, they’d been measuring the thickness of the ozone layer in the upper atmosphere, the layer of gas that deflects much of the sun’s radiation. Starting in the 1970s, it had started plummeting. By the mid-1980s, they observed that it was on track to be wiped out in the next few decades.
Their discovery was cause for worldwide alarm and unprecedented action. In short order, the international community marshaled its resources — scientific, economic, diplomatic — to mount a campaign to ban the chemical that caused the damage, chlorofluorocarbons (CFCs), and to restore the ozone layer.
Fast-forward to today: The ozone is on the path to recovery, if not fully restored. That progress hasn’t been without setbacks. The ozone hole is shrinking on average, but some years are bad ones — the hole was notably larger in 2020, following a 2019 when it was unusually small. Researchers have also raised suspicions that the rate at which atmospheric CFCs are falling suggests not all signatories to a treaty banning new production of CFCs are abiding by the agreement. And there have been unintended consequences in phasing out CFCs with a different chemical that has hurt our fight against climate change (more on this below).
But the damage we wrought last century has been reversed. Even with the complications and caveats, the world’s response to the ozone crisis should be seen as an instructive, even inspiring, success story — one that can perhaps inform our response to the climate crisis.
That’s the thrust of this year’s Future of Life Award from the Future of Life Institute, a nonprofit that studies how to reduce risks to our world. The 2021 award, handed out last month, went to three people who played a significant role in our triumph over the depletion of the ozone layer: atmospheric chemist Susan Solomon, geophysicist Joseph Farman, and Environmental Protection Agency official Stephen Andersen.
The award, which comes with a $50,000 prize for each recipient, is given to unsung heroes who made our world safer from existential or global catastrophic risks. Last year, the institute gave its award to William Foege and Viktor Zhdanov, who played key roles in the smallpox eradication fight. The previous year, it went to Matthew Meselson for his work on the Biological Weapons Convention.
This year’s award harks back to a crisis that unnerved — and galvanized — humanity in the 1980s and ’90s. The ozone layer reduces how much radiation makes it to the surface of the Earth. Without it, sunshine would be significantly deadlier to life on the planet. The primary culprit for its thinning, researchers discovered, was CFCs, a chemical compound that was present in everything from aerosol cans to refrigerators to solvents. As CFCs degrade in the upper atmosphere, they can break down ozone.
“Projections suggested that the ozone layer would collapse by 2050,” the Future of Life Institute’s Georgiana Gilgallon told me. “We’d have collapsing ecosystems, agriculture, genetic defects.” The sudden plunge in atmospheric ozone heralded a coming disaster.
But the world responded. With consumer boycotts, political action, a major international treaty called the Montreal Protocol, and a huge investment in new technologies to replace CFCs in all their commercial and industrial uses, new CFC production was brought effectively to a halt over the 1990s and early 2000s. It took a while to phase out existing devices that used CFCs, but CFC emissions have been steadily falling since the protocol went into effect.
“We see this as potentially the first instance in which humanity recognized and addressed a global catastrophic risk,” Gilgallon told me. There is still much to be done and some new problems to contend with, but measurements from the present day make it clear that the process of healing the ozone layer is well underway.
Ozone is a molecule made up of three oxygen atoms. (The oxygen we breathe is made up of just two.) There’s not much ozone floating around in the layer of atmosphere that we breathe — a good thing, since it’s actually a lung irritant and linked with respiratory disease.
But there’s a lot of it in the stratosphere (comparatively speaking, at least; it’s still only a tiny fraction of the overall air). It’s that layer of ozone that absorbs ultraviolet (UV) radiation, especially the specific wavelengths called UV-B.
UV-B radiation is what causes sunburns, and in high concentrations it causes more problems than that. It can lead to many kinds of cancer by damaging our DNA; most plants and animals also suffer when growing in a high-UV-radiation environment.
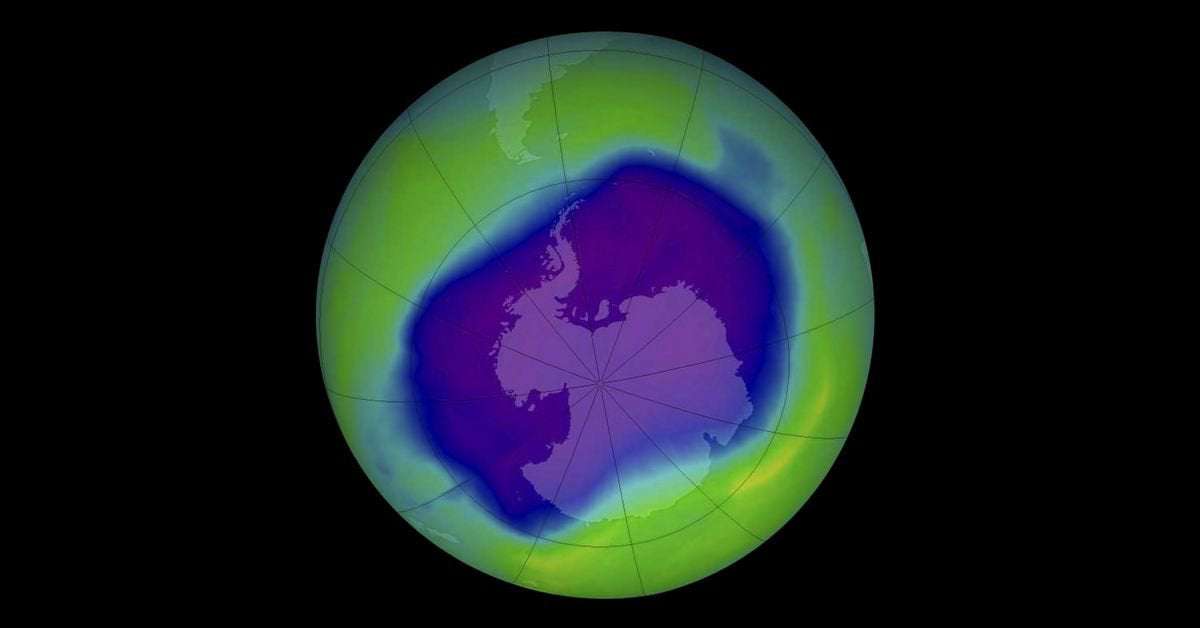
In the 1970s, researchers noticed that the ozone layer had started thinning, especially around the poles. (With the ozone layer constituting only about three in a million atoms in the stratosphere in the first place, “hole” is technically a misnomer — the “ozone hole” was really just an area where ozone levels had dropped by more than 30 percent in a decade.)
By the time the thinning of the ozone layer was measured, researchers Mario Molina and Sherry Rowland had already established the probable cause: CFCs.
CFCs were everywhere, and as far as everyone knew, they were the perfect chemical: nonreactive, cheap, and highly effective in a wide variety of manufacturing applications. They were building up in the atmosphere, but it was thought that since they were nonreactive, it couldn’t be a problem.
Molina and Rowland realized that that assumption was wrong. There’s a (possibly apocryphal) story of Rowland’s wife asking him how his work was going, and Rowland responding, “Well, the work is fantastic, but I think the Earth is ending.”
The problem was that CFCs break down in the upper atmosphere. And the chlorine in CFCs was actually reactive, binding with ozone to make oxygen and chlorine monoxide.
Molina and Rowland’s 1974 paper in Nature laying out the problem prompted discussion and debate, and environmental activists started pushing for change. But it didn’t move governments to coordinated international action. At the time, the exact implications of Molina and Rowland’s theory were hotly contested. Many researchers believed that ozone depletion would be a problem only on a time scale of centuries. There were some early worrying measurements that were dismissed as flukes.
What the measurements in the Antarctic taken a decade later showed definitively is that it was happening much, much faster than that. “Sometime around the late ’70s, it started dropping like a rock — [there] was more ozone depletion than Molina and Rowland had ever imagined,” Solomon said.
The fight in the 1980s against the depletion of the ozone layer had several stages that might seem familiar to those trying to unite the world to combat other problems.
First, there was the challenge of determining that there was in fact a threat and that CFCs were the cause. The initial work there was done by Molina and Rowland. But from the 1985 measurements taken by Joseph Farman — a geophysicist at the British Antarctic Survey — and his colleagues, it looked like the ozone layer was vanishing much faster than their models predicted.
Susan Solomon was the lead researcher on the team that figured out how the chlorine from CFCs was breaking down so much ozone. In 1986 and 1987, she led the National Ozone Expedition to Antarctica to collect the evidence that would confirm her theory. Scientists had originally thought that, while chlorine would interact with ozone, the process was naturally limited — after all, there weren’t that many atoms of chlorine loose.
Solomon and her team claimed that the process by which chlorine broke down ozone actually wasn’t as limited as initially thought and that the ozone breakdown could quickly spiral out of control: The chlorine monoxide that formed from chlorine’s interaction with ozone would then break down, releasing the chlorine atom to go break down more ozone.
“You can destroy hundreds of thousands of ozone molecules with one chlorine atom from a CFC molecule in the timescale that this stuff is in the stratosphere,” Solomon said.
The next stage of the fight was then convincing the world to do something about the problem. In 1986, UN negotiations began on a treaty to ban substances that reacted with ozone in the upper atmosphere, mainly CFCs. Stephen Andersen, at the time an official in the US Environmental Protection Agency, was a major figure in the negotiations. “He really made it happen,” the Future of Life Institute program director David Nicholson says.
The Montreal Protocol on Substances that Deplete the Ozone Layer was agreed upon and opened for signature in 1987. It went into force in 1989. Countries gradually began phasing out CFCs. Andersen’s team, Nicholson says, “systematically identified hundreds of solutions for phasing out CFCs from hundreds of industry sectors,” making it possible to shift manufacturing processes worldwide to chemicals that weren’t ozone-depleting.
Those chemicals in some cases have presented their own problems. For refrigerants, the world shifted to hydrofluorocarbons (HFCs), which endanger the ozone layer much less. Like the CFCs they replaced, though, HFCs are potent greenhouse gases — thousands of times more effective than carbon dioxide at trapping heat in our atmosphere. Twenty years ago, HFCs were an environmental step forward, allowing us to phase out CFCs. Today, policymakers and scientists are trying to phase out HFCs as well. Human ingenuity can solve our problems, but it can also create new ones as it does.
But in terms of the primary goal — healing the ozone layer — the worldwide effort was a huge success. CFC consumption declined from over 800,000 metric tons in the 1980s to an estimated 156 metric tons in 2014. Experts estimate that by 2050, the ozone layer will be back to the state it was in 1980.
And keeping the ozone intact buys us time in the fight against climate change. Yes, HFCs are a potent greenhouse gas. But CFCs contributed to global warming as well: They were powerful greenhouse gases in their own right, and by destroying the ozone layer, they contributed to warming by allowing more energy to reach the planet’s surface. One study found that ozone-depleting chemicals drove half of Arctic warming in the 20th century.
With that said, HFCs are still a big climate problem. In recent years, governments have been working to extend the hugely successful Montreal Protocol to phase them out too. It’s fair to say that, in some ways, the global fight against the ozone crisis was a complicated story, one that continues to be written.
But in other ways, it does offer some bracing clarity. The sheer speed with which the world went to work and enacted a global treaty to address a pressing environmental problem is, to contemporary eyes, downright bewildering. To a public accustomed to decades-long stalemates over climate policy, hearing how countries quickly lined up to sign an accord to save the planet may feel almost like a rebuke of our failures.
In many ways, the international community of the 1980s had an easier problem. CFCs were industrially useful, but there were substitutes; cost-effective substitutes for fossil fuels are coming into production now, decades into the climate crisis, but they certainly didn’t exist when we first started addressing it.
Politicians were more united in addressing the ozone layer than they’ve proven in addressing climate change. The Senate ratified the Montreal Protocol 83–0. Margaret Thatcher, not generally known for her friendliness to regulation, was a leader in the push for the Montreal Protocol and the effort to enable compliance by poor countries.
By contrast, politicians today (especially in the US) are fiercely divided over the proper government role in ending climate change, and the public is divided along partisan lines as well.
The picture we’re left with by the fight to heal the ozone layer is that specific individuals played a huge role in changing humanity’s trajectory but they did that mostly by enabling public activism, international diplomacy, and collective action. In the fight to improve the world, we can’t do without individuals and we can’t do without coordination mechanisms. But we should keep in mind how much we can do when we have both.
Coolguy6979 on October 16th, 2021 at 16:27 UTC »
This is an example of what can be achieved if all the nations come together to fight environmental issues like these. If only all nations could come together to fight off climate change…
wahchewie on October 16th, 2021 at 13:12 UTC »
Hang on what, I saw an article on here just a few weeks ago that the hole was as big as the 90s. So which one is it
bagsofcandy on October 16th, 2021 at 12:20 UTC »
What excellent news! We should plan one huge earth day celebration for it.