Culture condition for construction of contractile bovine muscle tissue
To construct contractile bovine muscle tissue, we cultured bovine myocyte-laden hydrogel on a culture device consisting of anchors with pillars fabricated using stereolithography and a substrate consisting of polydimethylsiloxane (PDMS) (Supplementary Fig. 1). After the anchors were integrated with the substrate, the pillars were arranged to protrude from the surface of the substrate. Bovine myocytes used for the tissue construction were prepared by culturing cells collected from commercial fresh beef. To evaluate proportion of myocytes in the collected cells, we carried out flow cytometry and found that >85% cells were myocytes (myoblasts and satellite cells) (Supplementary Fig. 2). The pillars were embedded in the hydrogel by gelling the hydrogel solution containing the bovine myocytes on the substrate. When the myocytes were cultured in the hydrogel for 14 days, fibre-shaped bovine muscle tissue of diameter 295 ± 105 µm (mean ± s.d., n = 32) was generated, the ends of which were immobilised with pillars, showing that the length of the muscle tissue was equal to the gap between the anchors (7 mm).
To compare the contractility of the cultured bovine muscle tissue prepared using different types of hydrogel, we used collagen and a mixture of fibrin and matrigel for tissue fabrication. In addition, to determine the effects of electrical stimulation on muscle tissue during culture, we stimulated the muscle tissue with electrical pulses (frequency: 1 Hz, duration: 2 ms, electrical field: 3 V/mm) for 2 h per day from day 3 to day 14; the muscle tissue was also cultured without the electrical pulses. As the result, we found that in the case of bovine muscle tissue formed using the fibrin-matrigel mixture, 100% muscle tissue stimulated with the electrical pulses (N = 9) and 56% muscle tissue cultured without the electrical pulses (N = 9) contracted according to the applied electrical pulses. In contrast, in the tissue formed with collagen, only 33% muscle tissue stimulated with the electrical pulses (N = 6) contracted according to the electrical pulses, whereas muscle tissue cultured without the electrical pulses (N = 6) did not contract (Fig. 2a). Furthermore, we confirmed that the contractile distance of the fibrin-matrigel-based muscle tissue cultured with the electrical stimulation was larger than that of the collagen-based muscle tissue (Fig. 2b) (Supplementary videos 1, 2). These results indicate that the use of fibrin-matrigel, combined with culture under electrical stimulation, facilitates construction of contractile bovine muscle tissue.
Fig. 2: Morphological and functional analysis of bovine muscle tissue. a Rate of fibre-shaped bovine muscle tissues capable of contracting in response to applied electrical stimulation (the number of contractile tissue/the number of all tissues), formed with the collagen (Col)-based tissues cultured with and without electrical stimulation (ES) (amplitude: 3 V/mm, frequency: 1 Hz, duration: 2 ms) (N = 6), and the fibrin-matrigel (Fib-Mat)-based tissues cultured with and without ES (N = 9). b Temporal variation of contractile distance of the fibre-shaped bovine muscle tissue formed with collagen and Fib-Mat depending on the ES. c Confocal images of the bovine muscle tissue; cell nucleus (blue), α-actinin (green). d Occupancy of myotubes in the short-axial cross-section of the muscle tissue under different culture conditions (n = 3). e Rate of myotube formation with α-actinin striped patterns in all myotubes in the muscle tissue. (n = 3) f Directional distribution of brightness calculated from FFT images corresponding to confocal images. 0 degree and 180 degree show short-axial direction of the tissue and 90 degree shows its long-axial direction. Black, red and blue lines in each plot represent values for different tissues. All error bars show standard deviation. Scale bar, (c) 50 μm. Full size image
We performed α-actinin immunostaining to evaluate the maturation of bovine myotubes differentiated from the bovine myocytes in muscle tissue. The confocal images of the immunostained muscle tissue formed by culturing myocytes in fibrin-matrigel structure with electrical stimulation showed that multiple myotubes possessed striped patterns of α-actinin, indicating formation of sarcomeres in the myotubes (Fig. 2c). Using confocal imaging, we investigated the occupancy rate of myotubes and rate of formation of myotubes containing stripe patterned α-actinin in the bovine muscle tissues. The short-axis sectional images confirmed that the occupancy rates of myotubes were 11% and 17% in muscle tissues constructed using collagen and fibrin-matrigel, respectively. The occupancy rates increased by approximately twofold after culturing myocytes with electrical stimulation; 20% and 31% of the muscle tissues were constructed with collagen and fibrin-matrigel, respectively (Fig. 2d). In addition, the fibrin-matrigel-based muscle tissue cultured with electrical stimulation showed the highest rate (50%) of myotubes containing stripe patterned α-actinin among the experimental group (Fig. 2e). These results indicate that the rate of myotube formation improves when culturing myocytes in fibrin-Matrigel with electrical stimulation. Furthermore, the confocal images of the immunostained muscle tissue showed alignment of myotubes in the tissue. To quantitatively assess the orientation of the myotubes, we calculated directional distribution of the myotubes from fast Fourier transform (FFT) images based on the immunostaining images (Fig. 2f). This result indicates that the myotubes were aligned to the long axis of the tissue irrespective of the hydrogel composition and with or without electrical stimulation. The morphological evaluations also indicate that the combination of culture in fibrin-Matrigel with electrical stimulation promoted the maturation of bovine muscle tissue containing aligned myotubes.
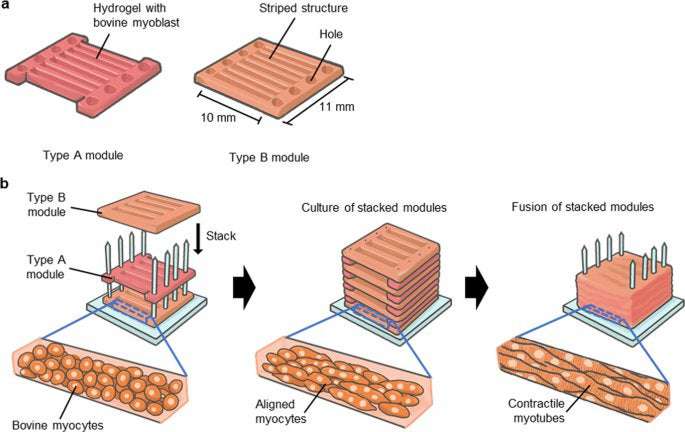
Construction of millimetre-thick bovine muscle tissue
For the construction of millimetre-thick bovine muscle tissue with highly aligned myotubes, we assembled myocyte-laden modules prepared using a modified version of previous methods.13,14 In this method, we prepared bovine myocyte-laden collagen modules shaped with a PDMS stamp. Upon culturing 40 assembled modules, the ends of which were immobilised to pillars, the modules containing 1-mm wide striped structures arranged parallel to each other at 0.3 mm intervals fused into one muscle tissue after 7 days of culture, resulting in the construction of millimetre-thick bovine muscle tissue of 8 mm × 10 mm × 7 mm (width×length×height) dimensions (Fig. 3a). To confirm integration of the striped structures in the millimetre-thick tissue, we prepared sectional images of the cultured muscle tissue stained with haematoxylin and eosin (H&E) (Fig. 3b). The short-axial sectional images showed that the myotubes were uniformly distributed, and that most of the striped structures were integrated into a single tissue. In the case where modules with 1-mm wide striped structures were arranged at 1 mm intervals following the width-height ratio (1:1) mentioned in previous studies on rat myoblasts,13 millimetre-thick muscle tissue was not formed, as the striped structures did not contact each other (Supplementary Fig. 3). This showed that the widths of the intervals should be smaller than the widths of the stripe structures for construction of millimetre-thick bovine muscle tissue.
Fig. 3: Morphological analysis of the millimetre-thick bovine muscle tissue. a Images of the millimetre-thick bovine muscle tissue on day 1 and day 7 of culture. The change in colour from Day 1 to Day 7 could be caused by the colour change of the phenol red in the culture medium or difference of the light exposure conditions when taking the photos. b Long-axial sectional image and short-axial sectional image of the muscle tissue stained with haematoxylin and eosin (H&E). c Directional distribution of brightness calculated from FFT images that corresponded to confocal images taken from a bovine muscle tissue constructed by stacking five myocyte-laden hydrogel modules. 0 degree and 180 degree show short-axial direction of the tissue, and 90 degree shows its long-axial direction. d Top and lateral views of the millimetre-thick bovine muscle tissue after release from the pillars. e The millimetre-thick bovine muscle tissue coloured using red food colouring agent. Scale bars, (a) 5 mm, (b) 0.2 mm (long-axial sectional image), 2 mm (short-axial sectional image), (d) 5 mm and (e) 1 cm. Full size image
We assessed the orientation of the myotubes in the millimetre-thick bovine muscle tissue from the H&E staining images. The long axis sectional images showed that myotubes were aligned to the longitudinal direction of the muscle tissue. (Fig. 3b). For quantitative evaluation of myotube orientation in muscle tissue, we prepared a cultured bovine muscle tissue consisting of five modules to visualise α-actinin using immunostaining, as observation inside immunostained millimetre-thick muscle tissue is difficult due to poor light transmission. Directional distribution of myotubes in the muscle tissue also showed that myotubes were oriented to the longitudinal direction of the tissue (Fig. 3c). From these results, we believe that the myocyte-laden module assembly method is useful for the construction of large bovine muscle tissue containing highly aligned myotubes. In addition, from the short-axial sectional image, enucleated cells were not observed, suggesting the maintenance of cellular viability without central necrosis in the tissue.
Furthermore, we assessed the feasibility of using the millimetre-thick bovine muscle tissue as cultured steak meat. Although the constructed muscle tissue was immobilised with pillars, we observed that the muscle tissue was easily released from the pillars using tweezers (Fig. 3d). Even after the muscle tissue was released from the pillars, the striped structures were maintained in the integrated state without being separated. In addition, after colouring the muscle tissue using red food colouring agent, we successfully obtained millimetre-thick bovine muscle tissue showing real meat-like appearance (Fig. 3e) (Supplementary video 3).
Characterisation of millimetre-thick bovine muscle tissue as food
To assess the difference in texture between the cultured millimetre-thick bovine muscle tissue and commercially available steak, we measured their breaking force as an index of stiffness for food.15 The cultured tissue and commercially available beef tenderloin were placed in a warm bath at 70 °C for 1 h for preparing experimental samples for the measurement (Fig. 4a). The weight loss of the commercial beef tenderloin after heating was 40% (weight before heating: 0.52 g, weight after heating: 0.31 g), whereas that of the cultured tissue was 90% (weight before heating: 0.31 g, weight after heating: 0.03 g), indicating that the cultured tissue contained considerable amounts of water. In contrast, the collagen structures without any myocytes were completely melted during the heating procedure, suggesting that interactions of myocytes with collagen could change thermal responsiveness of collagen. Furthermore, we measured the breaking force using the heated samples. The breaking force of the cultured tissue on day 14 was closer to that of the beef tenderloin than to that of the cultured tissue on day 4 (Fig. 4b). This result showed that the cultured millimetre-thick bovine muscle tissue hardened with culturing, indicating that the morphological changes accompanying the culture affected the stiffness of the cultured tissue.
Fig. 4: Food feature analysis of the large bovine muscle tissue. a Images of the millimetre-thick bovine muscle tissue and commercially available bovine beef tenderloin before and after heating (70 °C, 1 h). b Load at break of the muscle tissue and commercially available bovine beef tenderloin (n = 1). Scale bars, (a) 0.5 cm. Full size image
One of the advantages of the millimetre-thick bovine muscle tissue as cultured steak meat over commercial meat is its sterility, owing to control over the culture environment.16 We evaluated the microbial contamination of the muscle tissue to confirm this. Results of the general viable bacteria test showed that the amount of general bacteria in the muscle tissue cultured for 14 days was below the detection limit (<5 cfu/g), whereas that in the beef tenderloin was 1.7 × 105 cfu/g. This result clearly shows that the cultured mm-thick bovine muscle tissue was cleaner than general meat in terms of microbial contamination.
thr33pwood on March 2nd, 2021 at 14:56 UTC »
What do they use as a growth medium? It's been a while since I've done cell cultures but the medium I used to grow my cells in was fetal bovine serum - not exactly vegan stuff.
DodgyQuilter on March 2nd, 2021 at 14:36 UTC »
Start churning out lab raised pangolin, bear bile, tiger penis and every other Endangered Animal product that's being traded illegally. Flood the markets. Destroy the illegal trades.
wookietim on March 2nd, 2021 at 13:05 UTC »
I wonder - does lab grown meat remove the ethical argument for vegetarians?