The present study demonstrates the first attempt to assess the acute effects of psilocybin on glutamate levels in key areas of the human brain, which may play a major role in the actions of serotonergic psychedelics. Using an ultra-high field multimodal MRI approach, we demonstrated that, compared with placebo, psilocybin-induced region-dependent alterations in neurometabolite concentrations. Specifically, participants who received psilocybin demonstrated higher relative glutamate concentration levels in the mPFC, and lower relative glutamate concentration levels in the hippocampus. Analyses indicated that region-dependent alterations in glutamate were also correlated with different dimensions of ego dissolution. Whereas changes in mPFC glutamate were found to be the strongest predictor of negatively experienced ego dissolution, changes in hippocampal glutamate were found to be the strongest predictor of positively experienced ego dissolution.
Previous studies have demonstrated that the mPFC is highly enriched with 5-HT 2A receptors located primarily on layer V pyramidal neurons [61], and modulate excitatory transmission in cortical circuits [43, 62, 63]. Preclinical studies have demonstrated that activation of such receptors via serotonergic psychedelics results in a predominantly excitatory response [18, 64] via an increase in glutamate release, as observed in humans for the first time in this study. A glutamatergic increase in this area is also in accordance with human functional imaging studies which have demonstrated a hyperfrontal regional cerebral blood flow (CBF) pattern after psilocybin [46, 65], and similar 5-HT 2A agonist psychedelics [66, 67]. However, we also found that psilocybin administration was associated with higher levels of GABA in this area, results in line with findings that 5-HT 2A receptors are also located on GABAergic interneurons [17, 68]. Taken together, findings suggest that activation of 5-HT 2A receptors in the mPFC results in both excitation and inhibition of cortical pyramidal cells [17], potentially resulting in an increased metabolic rate in this area, but not necessarily increased neural input or output.
In contrast to the mPFC, the present study demonstrated that participants who received psilocybin demonstrated lower relative glutamate concentrations in the hippocampus, suggesting that psilocybin decreases glutamate in this area. Such a decrease is in line with data from a recent functional imaging study with psilocybin, demonstrating reduced absolute CBF in the hippocampus compared with placebo [69], of which the authors proposed two potential mechanisms. Namely, decrements could be due to agonism of 5-HT 2A receptors located on GABAergic interneurons [44], which can indirectly inhibit pyramidal neurons, decreasing activation in this area. Conversely, it has also been established that, along with the 5-HT 2A receptor, psilocin also has a high affinity for the 5-HT 1A receptor [70, 71]. Referred to as serotonin’s principal inhibitory receptor [72], the 5-HT 1A receptors highest density is found in the limbic regions of the brain such as the hippocampus [73] where it is expressed on neurons that are postsynaptic to the serotonergic input. Thus lower levels in glutamate as seen in this study, as well as regional decreases reflected in others [69], could be due to activation of postsynaptic inhibitory 5-HT 1A receptors. Nevertheless, due to methodological limitations, this study is not able to delineate which mechanism is contributing to the lower levels in glutamate. Further information could have been potentially gained from quantification of GABA in the hippocampus, however we were unable to reliably do so, due to inherent quantification challenges when assessing GABA levels, arising from low brain concentration levels, metabolite signal overlap, and low signal-to-noise in the hippocampus [74, 75]. Future studies with sequences developed to specifically quantify GABA in low signal-to-noise areas should make further attempts to do so, given recent research implicating hippocampal GABA in the pathology of disorders that psychedelics are being investigated to treat [76].
In the current study, psilocybin induced previously established key features of a psychedelic experience: increases in feelings of ego dissolution, and disrupted RSN activity. Psilocybin increased scores on all dimensions of the 5D-ASC [16], as well as on the EDI [1]. In addition, psilocybin altered within-network FC similarly as has been shown with LSD, including decrements in coactivation within the DMN, visual network 1, and the auditory network [32, 33]. Finally, we demonstrated higher between-network FC across all networks, which is similar with previous studies assessing the same after psilocybin [35, 42] and LSD [32, 33].
Finally, we assessed the relationship between psilocybin-induced changes in the brain, and the subjective experience of sense of self. Canonical correlations were conducted to predict increases in ratings of AED, the dimension encompassing the loss of autonomy and self-control of thought processes, intentionality, decision making, and spontaneous movements [46]. Our data support the conclusion that increasing levels of mPFC glutamate were the strongest predictor in regards to feelings of AED, with decreasing anterior DMN FC and hippocampal glutamate being secondary predictors. These findings are in line with previous work, implicating increased frontal metabolism in feelings of AED after psilocybin [46] and ego pathology in the ketamine model of psychosis [77]. Interestingly, AED-associated changes in mood include paranoia, heightened arousal and attention to the surroundings, and anxiety [46]. A paradoxical effect of serotonergic psychedelics is that acutely they have been found to increase feelings of anxiety [6, 78], whereas clinical trials with psychedelic drugs suggest long-term anxiety relief in patients [11, 12, 14]. Accordingly, there is a wide range of animal and human pharmacological evidence supporting the role of the glutamatergic system in anxiety [79], with increases in glutamate in the frontal cortex associated with high versus low state-trait anxiety [80], and reductions corresponding to anxiety-related symptomatic relief [81]. Taken together, the finding that mPFC glutamate was by far the strongest predictor of increased feelings of anxiety, one could propose that acute psychedelic-induced anxiety may be due to localized glutamate-induced hyperfrontality, whereas long-term reductions could be due to agonist-induced 5-HT 2A receptor downregulation in this area [72, 82]. Nonetheless, future studies should assess long-term changes in 5-HT 2A receptor function in the mPFC, and their relation with subjective effects.
We also assessed the relationship between psilocybin-induced brain changes and feelings of positively experienced ego dissolution, including ratings on the EDI, and scores of OB on the 5D-ASC. We found that the primary predictor of positively experienced ego dissolution was a decrement in hippocampal glutamate, with secondary contributions of mPFC glutamate and posterior DMN integrity. Previous work has implicated both the MTL (containing the hippocampus) and DMN circuitry in the neural correlates of the self [49]. Namely, abnormal function of MTL regions have been implicated in psychotic states [83, 84] and feelings of depersonalization [85] and ego-disturbances [86]. Similarly, studies of drug-induced ego dissolution have found that the decoupling of MTL regions such as the parahippocampus and the DMN correlate positively with feelings of ego dissolution [49, 87], with this decoupling being hypothesized to be one of the main underlying mechanisms of the subjective experience [47,48,49]. In regards to why this gives rise to ego dissolution, it has been suggested that psychedelic drug-induced decoupling of these regions results in a temporary loss of access of semantic autobiographical information, resulting in a breakdown of one’s personal identity [87]. Our data add to this hypothesis, suggesting that modulations of hippocampal glutamate in particular may be a key mediator in the decoupling underlying feelings of (positive) ego dissolution. Interestingly, although the DMN has been the most implicated RSN in this process, Lebedev et al. [49] found that increases in ego dissolution correlated with decreased FC between the parahippocampal formation and other major networks, such as the salience, frontoparietal, and sensorimotor network; suggesting a key role in this area in particular, as our data also demonstrate. However future research should further assess the contribution of other areas to this experience, such as the posterior cingulate cortex.
Implications of these findings also extend far beyond understanding the neurobiology of the acute psychedelic experience and drug-induced ego dissolution. There is growing evidence that psychedelics can provide therapeutic relief for individuals suffering from increasingly common and difficult to treat disorders such as depression, anxiety, addiction, and post-traumatic stress disorders [4, 9, 11, 88, 89]. Thus understanding the mechanisms by which psychedelics provide symptomatic relief may identify novel therapeutic targets. Interestingly, the degree of ego dissolution has been found to correlate with long-term clinical outcomes [90] and increases in well-being [10, 91]. In addition, a hypothetical (neurobiological) model has been proposed to explain the long-term effects witnessed in clinical trials. It has been suggested that indirect activation of glutamate networks via 5-HT 2A receptor agonism increases BDNF, and ultimately enhances neuroplasticity [16]. In line with this, it has been shown in preclinical models that psychedelics increase functional and structural neuroplasticity [92], however evidence in humans is limited, due to restrictions of methodological techniques. Our data provide indirect evidence that psychedelics might have the potential to increase neuroplasticity in the human cortex via increased glutamatergic activity, but not in the hippocampus; findings that are in accordance with previous 5-HT 2A receptor activation studies [27, 93, 94]. In addition, psilocybin administration was associated with higher levels of mPFC NAA, a compound regarded as a measure of neuronal viability and function, and decreased in disorders associated with regional neuronal loss and disrupted neuronal function [95].
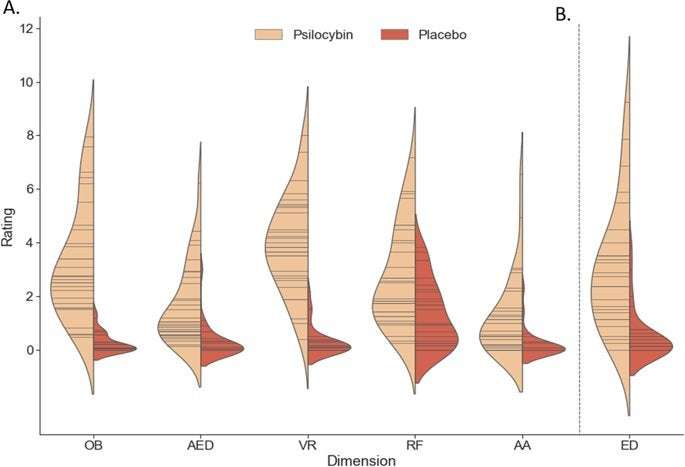
Of note, compared to previous psychedelic studies, the dose administered was low to moderate [96], and thus not high enough to induce total ego dissolution. However, the aim of this study was not to assess maximal effects of psilocybin, but rather an effective dose that would induce a relevant psychedelic state that participants could endure in the MRI scanner. Our data demonstrate that the dose was effective, inducing significantly higher levels of both positively and negatively experienced ego dissolution compared with placebo, as well as the other subjective effects representative of a psychedelic state (Figs. 1, S1). Furthermore, although BOLD sensitivity is increased by the use of ultra-high magnetic fields, geometric distortions become more prominent, which could have affected our BOLD signal in inferior brain regions [97], and our scan time was arguably short from a test–retest reliability standpoint [98]. Nevertheless, our results are similar to aforementioned studies [32, 33, 35, 42] who acquired their data at a lower field strength, with varying scanning lengths. Finally, an inherent difficulty of studying substances with such salient subjective effects is maintaining the treatment blind. Thus, it could be suggested that participant recognition of the treatment condition could affect neural and subjective results, emphasizing the importance of active placebo conditions or cross-psychotropic comparisons in future trials.
In conclusion, our data demonstrate that the serotonergic psychedelic, psilocybin, acutely induces region dependent alterations in glutamate that correlate with established behavioral changes during the psychedelic state. Such findings provide further insights into the underlying neurobiological mechanisms of the psychedelic state, and importantly, provide a neurochemical basis for how these substances alter individuals’ sense of self, and may be giving rise to therapeutic effects witnessed in ongoing clinical trials.
kinakomochidayo on May 27th, 2020 at 20:02 UTC »
I'm kind of hoping this puts the nail in the coffin with researchers trying to separate the psychedelic effects from the anti-depressant effects so that they can turn it into some kinda pill people have to take every day.
tumeric7890 on May 27th, 2020 at 17:31 UTC »
Hope this becomes utilised more in sectors such as health psychology for helping cope with long-term illnesses/ addiction etc. The results of the research so far have been mind-blowing.
zwis99 on May 27th, 2020 at 17:30 UTC »
I wonder if this will lead to a better understanding of consciousness in general