The Ad5 vectored COVID-19 vaccine is tolerable and immunogenic at 28 days post-vaccination. Humoral responses against SARS-CoV-2 peaked at day 28 post-vaccination in healthy adults, and rapid specific T-cell responses were noted from day 14 post-vaccination. Our findings suggest that the Ad5 vectored COVID-19 vaccine warrants further investigation.
Between March 16 and March 27, 2020, we screened 195 individuals for eligibility. Of them, 108 participants (51% male, 49% female; mean age 36·3 years) were recruited and received the low dose (n=36), middle dose (n=36), or high dose (n=36) of the vaccine. All enrolled participants were included in the analysis. At least one adverse reaction within the first 7 days after the vaccination was reported in 30 (83%) participants in the low dose group, 30 (83%) participants in the middle dose group, and 27 (75%) participants in the high dose group. The most common injection site adverse reaction was pain, which was reported in 58 (54%) vaccine recipients, and the most commonly reported systematic adverse reactions were fever (50 [46%]), fatigue (47 [44%]), headache (42 [39%]), and muscle pain (18 [17%]. Most adverse reactions that were reported in all dose groups were mild or moderate in severity. No serious adverse event was noted within 28 days post-vaccination. ELISA antibodies and neutralising antibodies increased significantly at day 14, and peaked 28 days post-vaccination. Specific T-cell response peaked at day 14 post-vaccination.
We did a dose-escalation, single-centre, open-label, non-randomised, phase 1 trial of an Ad5 vectored COVID-19 vaccine in Wuhan, China. Healthy adults aged between 18 and 60 years were sequentially enrolled and allocated to one of three dose groups (5 × 10, 1 × 10, and 1·5 × 10viral particles) to receive an intramuscular injection of vaccine. The primary outcome was adverse events in the 7 days post-vaccination. Safety was assessed over 28 days post-vaccination. Specific antibodies were measured with ELISA, and the neutralising antibody responses induced by vaccination were detected with SARS-CoV-2 virus neutralisation and pseudovirus neutralisation tests. T-cell responses were assessed by enzyme-linked immunospot and flow-cytometry assays. This study is registered with ClinicalTrials.gov
Many vaccine candidates are in rapid development, including recombinant-protein based vaccines, replicating or non-replicating viral vector-based vaccines, DNA vaccines, and mRNA vaccines (which mostly have focused on the spike glycoprotein or receptor binding domain), live attenuated vaccines, and inactivated virus vaccines. All of these vaccine platforms have advantages and disadvantages, and it is too soon to predict which will be more successful. Our study suggests that there is potential for further investigation of the Ad5 vectored COVID-19 vaccine for prevention of COVID-19.
This first-in-human trial showed that the Ad5 vectored COVID-19 vaccine was tolerable and immunogenic in healthy adults. One dose of the vaccine at all dose concentrations (5 × 10 10 , 1 × 10 11 , and 1·5 × 10 11 viral particles) tested induced both specific antibody and T-cell responses in most participants. Rapid specific T-cell responses were noted at day 14 and specific humoral responses against severe acute respiratory syndrome coronavirus 2 peaked at day 28 post-vaccination. Although we found that the high dose vaccine tended to be more immunogenic than the middle dose and low dose vaccines, it was also associated with a higher reactogenicity. Severe fever, fatigue, dyspnoea, muscle pain, and joint pain were reported in some of the recipients in the high dose group.
We searched PubMed on May 8, 2020, for clinical trial reports with the terms “COVID-19” or “SARS-CoV-2”, “vaccine”, and “clinical trial” and no date or language restrictions; no other data from a human clinical trial of COVID-19 vaccine have been reported thus far, to our knowledge. We also searched the ClinicalTrials.gov registry for unpublished trials of COVID-19 vaccines, up to May 6, 2020. In addition to the adenovirus type-5 (Ad5) vectored COVID-19 vaccine reported here, seven candidate COVID-19 vaccines are in ongoing clinical trials, including Moderna's mRNA COVID-19 vaccine, Inovio Pharmaceuticals' DNA vaccine, Sinovac, Wuhan and Beijing Institute of Biological Products' inactive COVID-19 vaccines, University of Oxford's chimpanzee adenovirus-vectored vaccine, and BioNTech's mRNA COVID-19 vaccine.
In the absence of effective prevention measures, current management to control the epidemic is the enforcement of quarantine, isolation, and physical distancing.Effective vaccines against COVID-19 are urgently needed to reduce the enormous burden of mortality and morbidity associated with SARS-CoV-2 infection.There are more than 100 candidate vaccines in development worldwide,among them at least eight have started or will soon start clinical trials. These include Moderna's mRNA COVID-19 vaccine and CanSino's non-replicating adenovirus type-5 (Ad5) vectored COVID-19 vaccine, which both entered phase 1 clinical trials on March 16, 2020; Inovio Pharmaceuticals' DNA vaccine for COVID-19, which entered trials on April 3, 2020; three inactive COVID-19 vaccines manufactured by Sinovac, Wuhan Institute of Biological Products, and Beijing Institute of Biological Products entered clinical trials in April, 2020, successively; University of Oxford's non-replicating chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19, and BioNTech's mRNA COVID-19 vaccine also started trials in recent months.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in January, 2020. The virus is highly transmissible between humans and has spread rapidly, causing the COVID-19 pandemic.Patients infected with SARS-CoV-2, especially older patients and those with pre-existing respiratory or cardiovascular conditions are at greater risk for severe complications, including severe pneumonia, acute respiratory distress syndrome, multiple organ failure, and in some cases, death.By May 20, 2020, SARS-CoV-2 had infected more than 4·7 million people across 215 countries or territories and killed more than 316 000 worldwide.
The sponsors of the study participated in study design, but had no role in data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
An independent data and safety monitoring committee, with one independent statistician, one clinician, and one epidemiologist, was established before the start of the trial. Safety data for the first 3 days post-vaccination were assessed and reviewed by the committee to ensure that there was sufficient holding time between dose escalation. This study is registered with ClinicalTrials.gov
The sample size was not determined on the basis of statistical power calculations; however, a minimum sample size of 20–30 participants for a pilot vaccine trial has been recommended by the National Medical Products Administration, China. We assessed the number and proportion of participants with adverse reactions post-vaccination and compared safety profiles across the dose groups. The antibodies against SARS-CoV-2 were presented as geometric mean titres with 95% CIs and the cellular responses were shown as a proportion of positive responders. We used the χ 2 test or Fisher's exact test to analyse categorical data, ANOVA to analyse the log transformed antibody titres, and Wilcoxon rank-sum test for data that were not normally distributed. When the overall difference across the three groups was significant, pairwise comparisons were made and the differences between groups were estimated with 95% CIs. Multivariable analysis was used to establish the possible effects on the immunogenicity and safety profile of the vaccine candidates. Hypothesis testing was two-sided with an α value of 0·05. Statistical analyses were done by a statistician using SAS (version 9.4) or GraphPad Prism 8·0·1. SPICE (version 6.0) was used for the analysis of data from multicolour flow cytometry experiments.
We analysed all outcomes in the intention-to-treat cohort. The primary endpoint for safety was the occurrence of adverse reactions within 7 days after the vaccination. Any abnormal changes in laboratory measures at 7 days post-vaccination, and adverse events within 28 days across the treatment groups were also analysed as secondary safety endpoints. The specific ELISA antibody titres to RBD and the spike glycoprotein, and the neutralising antibody amounts against live SARS-CoV-2 and a pseudovirus were measured as humoral immunogenicity endpoints. We defined a positive antibody response (seroconversion) as at least a four-fold increase in post-vaccination titre from baseline. ELISpot IFNγ and positive T-cell responses measured by intracellular cytokine staining assays were compared across the groups as endpoints for cell-mediated responses. Stratified analyses of the immune responses were done based on the pre-existing Ad5 neutralising antibody titres among the participants as low or negative (≤1:200) or high (>1:200).
Blood samples were taken from participants for serology tests at the scheduled site visits before the vaccination, and on days 14 and 28 after the vaccination. We assessed binding antibody responses against the receptor binding domain (RBD) and spike glycoprotein with ELISA kits manufactured by Beijing Wantai BioPharm (Beijing, China). A dilution of 1:40 was the positivity cutoff value for ELISA. We also measured the neutralising antibody responses induced by vaccination using both live SARS-CoV-2 virus neutralisation (virus strain SARS-CoV-2/human/CHN/Wuhan_IME-BJ01/2020, GenBank number MT291831.1) and pseudovirus neutralisation tests (a vesicular stomatitis virus pseudovirus system expressing the spike glycoprotein).Peripheral blood mononuclear cells were isolated from whole blood before the vaccination and at days 14 and 28 post-vaccination. Specific T-cell responses were quantified with an interferon (IFN) γ enzyme-linked immunospot (ELISpot) assay using fresh peripheral blood mononuclear cells stimulated with overlapping spike glycoprotein peptide pools for about 12–24 h before detection, and expressed as the number of spot-forming cells per 100 000 cells. All measurements were subtracted from the unstimulated control values, and minus values were corrected to zero. The results were considered positive if there was at least a two-times increase in the number of IFNγ secreting T cells post-vaccination. We also assessed the CD4and CD8T-cell responses to vaccination according to the secretion of IFNγ, interleukin-2 (IL-2), and tumour necrosis factor α (TNFα), which were measured by intracellular cytokine staining assays in peripheral blood mononuclear cells after the stimulation with overlapping spike glycoprotein peptide pools for about 6 h and detected by flow cytometry. Similar methods to measure T-cell responses by intracellular cytokine staining assays have been reported previously.The pre-vaccination and post-vaccination anti-Ad5 neutralising antibody titres were detected with a serum neutralisation assay.
Adverse events were self-reported by the participants, but verified by investigators daily during the first 14 days after vaccination. Subsequently, adverse events were recorded by the participants on diary cards in the following weeks. Laboratory safety tests including white blood cell count, lymphocyte count, neutrophils, platelets, haemoglobin, alanine aminotransferase, aspartate aminotransferase, total bilirubin, fasting blood glucose, and creatinine were measured on day 7 to assess any toxic effects post-vaccination. We graded adverse events and abnormal changes in laboratory tests according to the scale issued by the China State Food and Drug Administration (version 2019).
A single shot was allocated intramuscularly in the arm of the participants in the low dose group, with one vial of the Ad5 vectored COVID-19 vaccine (5 × 10 10 viral particles per 0·5 mL). The participants in the middle dose group received one shot intramuscularly in the arm with two vials of the Ad5 vectored COVID-19 vaccine (1 × 10 11 viral particles per mL). Participants in the high dose group received a double-shot regimen with one vial of the Ad5 vectored COVID-19 vaccine in one arm and two vials of the Ad5 vectored COVID-19 vaccine in the other arm (1·5 × 10 11 viral particles per 1·5 mL).
The Ad5 vectored COVID-19 vaccine was developed by Beijing Institute of Biotechnology (Beijing, China) and CanSino Biologics (Tianjin, China). The vaccine is a replication defective Ad5 vectored vaccine expressing the spike glycoprotein of SARS-CoV-2. We cloned an optimised full-length spike gene based on Wuhan-Hu-1 (GenBank accession number YP_009724390) with the tissue plasminogen activator signal peptide gene into an E1 and E3 deleted Ad5 vector, and constructed the Ad5 vectored COVID-19 vaccine using the Admax system from Microbix Biosystem (Toronto, ON, Canada). The Ad5 vectored COVID-19 vaccine was manufactured as a liquid formulation containing 5 × 10 10 viral particles per 0·5 mL in a vial.
The protocol and informed consent were approved by the institutional review board of the Jiangsu Provincial Center of Disease Control and Prevention. Written informed consent from all participants was obtained before screening. This study was undertaken by Jiangsu Provincial Center for Disease Control and Prevention, Hubei Provincial Center for Disease Control and Prevention, and Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology in accordance with the Declaration of Helsinki and Good Clinical Practice.
We did a single-centre, open-label, non-randomised, dose-escalation phase 1 trial of an Ad5 vectored COVID-19 vaccine candidate in a rehabilitation centre in Wuhan, Hubei province, China. Eligible participants were healthy adults aged between 18 and 60 years, who did not have SARS-CoV-2 infection, confirmed by negative results of serum specific IgM and IgG antibodies with a commercial SARS-CoV-2 rapid test kit (Jinwofu, Beijing, China), negative nucleic acid for SARS-CoV-2 in pharyngeal swabs or sputum and anal swabs detected with a nucleic acid diagnostic kit (PCR-fluorescence probing, Sansure Biotech, Changsha, China), and a clear chest CT image with no evidence of lesions in the lungs at the time of screening. Exclusion criteria were a history of seizures or mental illness; allergy to any ingredient included in the vaccine; acute febrile disease on the day of enrolment; receipt of any blood products in the past 4 months; receipt of any research medicines or vaccine in the past month; and being unable to comply with the study schedule. Further details are outlined in the protocol.Participants were sequentially enrolled to receive a single intramuscular injection in a dose-escalating manner: the first group of participants were allocated to receive the low dose, followed up for a minimum of 3 days before proceeding to recruit further participants to receive the middle dose, and then after safety observation for 3 days, the last group of participants were recruited and allocated to receive the high dose. The administration of higher dose injections and new enrolment were paused if any criteria for pausing dose escalation were met.
To exclude any possible SARS-CoV-2 exposure during the study period, we tested the serum antibodies to nucleocapsid protein of SARS-CoV-2 in participants at day 28 using a special IgG/IgM rapid test kit (Vazyme Biotech, number CD101, Nanjing, China), but none of the participants were positive.
IFNγ was detected from CD4and CD8T cells after the vaccination at day 14 and 28, in all dose groups ( figure 2 appendix p 13 ). The TNFα expression from CD4T cells tended to be significantly lower in the low dose group than that in the high dose (p<0·0001) and middle dose groups (p=0·0032), on day 14. The TNFα expression from CD8T cells showed an overall p value of less than 0·0001 across the three groups on day 14. And the TNFα expression from CD8T cells tended to be higher in the high dose group than that in both the middle dose group (p=0·016) and the low dose group (p<0·0001). The p values are for the pairwise comparisons between groups. Amounts of IL-2 detected from CD4T cells were higher than that detected from CD8cells. The proportions of polyfunctional phenotypes detected from memory CD4T cells were higher than those from CD8T cells. Higher proportions of polyfunctional phenotypes were noted with the higher vaccine doses. We also noted that pre-existing Ad5 neutralising antibody had a negative effect on the pattern of T-cell responses ( appendix pp 14–16 ). A post-hoc analysis showed that 28 (78%) participants in the low dose group, 33 (92%) participants in the middle dose group, and 36 (100%) participants in the high dose group showed either positive T-cell responses to spike glycoprotein or seroconversion of neutralising antibody to live SARS-CoV-2, at day 28 post-vaccination ( appendix p 17 ).
(A) Percentage of cells secreting IFNγ, TNFα, and IL-2 from CD4 + T cells. (B) Percentage of cells secreting IFNγ, TNFα, and IL-2 from CD8 + T cells. (C) The proportion of CD4 + T cells and CD8 + T cells producing any combination of IFNγ, TNFα, and IL-2. The analyses are for 108 participants, with 36 in each dose group. IFN=interferon. TNF=tumour necrosis factor. IL=interleukin.
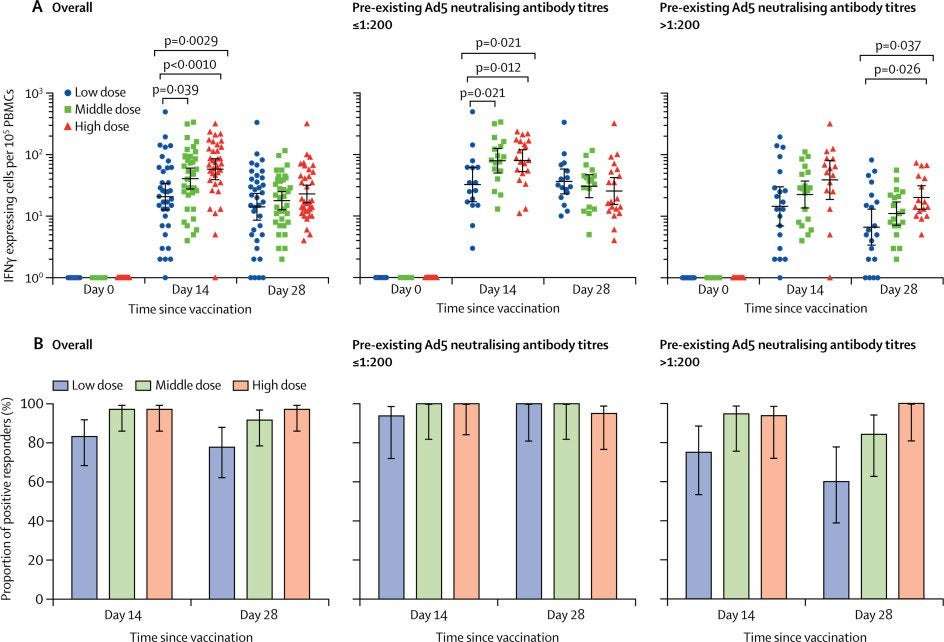
ELISpot responses at baseline were undetectable with spot-forming cells below the level of detection of the assay in all participants, but peaked at day 14 post-vaccination. The proportions of positive responders ranged from 83–97% across the dose groups, with a mean number of spot-forming cells per 100 000 cells of 20·8 (95% CI 12·7–34·0) in the low dose group, 40·8 (27·6–60·3) in the middle dose group, and 58·0 (39·1–85·9) in the high dose group ( figure 1 ). T-cell responses in the high dose group were significantly higher than that in the low dose group (p<0·0010), but not significant compared with that in the middle dose group. A slight decrease of the T-cell responses across the dose groups was noted at day 28. High levels of baseline Ad5 neutralising antibody titre reduced the peak of post-vaccination T-cell responses in all the dose groups, particularly for the low dose group. Despite the effect of high pre-existing Ad5 immunity, positive responders were identified in 15 (75%) of 20 participants in the low dose group, 18 (95%) of 19 participants in the middle dose group, and 15 (94%) of 16 participants in the high dose group at day 14, and 12 (60%) of 20 participants in the low dose group, 16 (84%) of 19 participants in the middle dose group, and 16 (100%) of 16 participants in the high dose group at day 28.
(A) The number of specific T cells with secretion of IFNγ at days 0, 14, and 28 in all participants, and stratified by pre-existing Ad5 neutralising antibody titres. (B) The proportion of positive ELISpot responders at days 14 and 28 post-vaccination in all participants, and stratified by pre-existing Ad5 neutralising antibody titres. IFN=interferon. PBMCs=peripheral blood mononuclear cells. Ad5=adenovirus type-5. ELISpot=enzyme-linked immunospot.
Before vaccination, 20 (56%) participants in the low dose group, 19 (53%) participants in the middle dose group, and 16 (44%) participants in the high dose group had a high pre-existing Ad5 neutralising antibody titre (>1:200). Only five (25%) participants of 20 in the low dose group, seven (37%) participants of 19 in the middle dose group, and ten (63%) participants of 16 in the high dose group, who had high pre-existing Ad5 immunity, had at least a four-fold increase in neutralising antibody titre at day 28 post-vaccination ( appendix pp 8–10 ). Multivariable analysis showed that high pre-existing Ad5 neutralising antibody titres compromised the seroconversion of neutralising antibody post-vaccination, regardless of the vaccine doses, and recipients aged 45–60 years seemed to have lower seroconversion of neutralising antibody compared with the younger recipients ( appendix p 11 ). The Ad5 neutralising antibodies were significantly boosted post-vaccination ( appendix p 12 ).
Rapid binding antibody responses to RBD were observed in all three dose groups from day 14 ( table 3 ). At day 28, the recipients in the high dose group tended to have a higher binding antibody geometric mean titre of 1445·8 (95% CI 935·5–2234·5), followed by 806·0 (528·2–1229·9) in the middle dose group, and 615·8 (405·4–935·5) in the low dose group (high dose vs low dose 1611·5, 531·5–2691·5). At least a four-fold increase of anti-RBD antibodies was noted in 35 (97%) of 36 participants in the low dose group, 34 (94%) of 36 in the middle dose group, and 36 (100%) of 36 in the high dose group. Neutralising antibodies against live SARS-CoV-2 were all negative at day 0, and increased moderately at day 14, peaking at 28 days post-vaccination. Neutralising antibody titre with a geometric mean titre of 34·0 (95% CI 22·6–50·1) was noted in the high dose group, which was significantly higher compared with 16·2 (10·4–25·2) in the middle dose group and 14·5 (9·6–21·8) in the low dose group, with an estimated difference of 27·7 (1·0–54·4) between the high dose group and the middle dose group and 33·2 (6·5–59·9) between the high dose group and the low dose group at day 28. Meanwhile, 18 (50%) participants in the low dose group, 18 (50%) in the middle dose group, and 27 (75%) in the high dose group had at least a four-fold increase in neutralising antibody titres by day 28. Similar patterns of the binding antibody to spike glycoprotein and neutralising antibody titre to pseudovirus post-vaccination across the dose groups were also noted ( appendix p 6 ). The association between the ELISA antibodies to RBD and neutralising antibody titres against live virus showed a moderate positive correlation of 0·749, and that between the ELISA antibodies to spike glycoprotein and neutralising antibody titres against live virus was 0·753, at the peak antibody response (p<0·0001). The neutralising antibody titres measured using a pseudovirus were also correlated well with those measured by live SARS-CoV-2 ( appendix p 7 ).
Data are mean (95% CI) or n (%). The p values are the result of comparison across the three dose groups. If the difference was significant across the three groups, the differences between groups were estimated with 95% CIs. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. GMT=geometric mean titre.
87 (81%) of 108 participants reported at least one adverse reaction within the first 7 days after the vaccination: 30 (83%) in the low dose group, 30 (83%) in the middle dose group, and 27 (75%) in the high dose group ( table 2 ). No significant difference in the overall number of adverse reactions across the treatment groups was observed. The most common injection site adverse reaction was pain, which was reported in 58 (54%) vaccine recipients. Pain was reported in 17 (47%) participants in the low dose group, 20 (56%) participants in the middle dose group, and 21 (58%) participants in the high dose group. The most commonly reported systematic adverse reactions overall were fever (50 [46%]), fatigue (47 [44%]), headache (42 [39%]), and muscle pain (18 [17%]). Fever was reported in 15 (42%) participants in the low dose group, 15 (42%) participants in the middle dose group, and 20 (56%) participants in the high dose group. Headache was reported in 14 (39%) participants in the low dose group, 11 (31%) participants in the middle dose group, and 17 (47%) participants in the high dose group. Muscle pain was reported in seven (19%) participants in the low dose group, three (8%) participants in the middle dose group, and eight (22%) participants in the high dose group. Most adverse reactions were mild or moderate in severity. Nine participants (two [6%] in the low dose group, two [6%] in the middle dose group, and five [14%] in the high dose group) had an episode of severe fever (grade 3) with axillary temperature greater than 38·5°C. Of them, one (3%) from the high dose group reported severe fever along with severe symptoms of fatigue, dyspnoea, and muscle pain. One participant in the high dose group reported severe fatigue and joint pain ( appendix p 3 ). These reactions occurred within 24 h post-vaccination, and persisted for no more than 48 h. We found no significant difference in the incidences of adverse reactions or overall adverse events among the dose groups. High pre-existing Ad5 immunity (titre of >1:200 vs ≤1:200) was associated with significantly fewer occurrences of fever post-vaccination (odds ratio 0·3, 95% CI 0·1–0·6; appendix p 4 ). No serious adverse event was reported within 28 days. At day 7 after vaccination, nine (8%) participants had mild to moderate total bilirubin increase, ten (9%) had alanine aminotransferase increase, and four (4%) had fasting hyperglycaemia ( appendix p 5 ), but no instances were considered as clinically significant.
Data are n (%). Any refers to all the participants with any grade adverse reactions or events. Adverse reactions and events were graded according to the scale issued by the China State Food and Drug Administration. Grade 3=severe (ie, prevented activity). GI=gastrointestinal.
Between March 16 and March 27, 2020, we screened 195 individuals for eligibility. Of them, 108 were sequentially enrolled and assigned to receive the low dose (n=36 [33%]), middle dose (n=36 [33%]), or high dose (n=36 [33%]) of the Ad5 vectored COVID-19 vaccine ( appendix p 2 ). All participants completed the vaccination and the scheduled visits within 28 days. Baseline characteristics of the participants were similar across the treatment groups ( table 1 ).
11 viral particles, presenting as severe fever, fatigue, muscle pain, or joint pain, which might be associated with viraemia caused by Ad5 vector infection. However, the severe adverse reactions were transient and self-limiting. Additionally, no abnormal changes in laboratory measurements were clinically significant or considered to be related to the vaccine. The profile of adverse events reported in this trial is similar to that of another Ad5 vector-based Ebola vaccine expressing glycoprotein. 16 Zhu FC
et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. 10 viral particles) and middle dose (1 × 1011 viral particles) to be further assessed in a phase 2 clinical trial. To our knowledge, this is the first report on a first-in-human clinical trial of a novel Ad5 vectored COVID-19 vaccine. The Ad5 vectored COVID-19 vaccine was tolerated in healthy adults in all three dose groups. The most common adverse reactions were fever, fatigue, headache, and muscle pain with no significant difference in the incidence of adverse reactions across the groups. Most adverse events reported were mild or moderate in severity. We noticed a higher reactogenicity profile of the high dose at 1·5 × 10viral particles, presenting as severe fever, fatigue, muscle pain, or joint pain, which might be associated with viraemia caused by Ad5 vector infection. However, the severe adverse reactions were transient and self-limiting. Additionally, no abnormal changes in laboratory measurements were clinically significant or considered to be related to the vaccine. The profile of adverse events reported in this trial is similar to that of another Ad5 vector-based Ebola vaccine expressing glycoprotein.To accelerate the process of clinical evaluation of the candidate COVID-19 vaccine, we selected doses for the phase 2 study mainly on the basis of the safety profile of the candidate vaccines shown in the participants within 7 days and 14 days post-vaccination. We chose the low dose (5 × 10viral particles) and middle dose (1 × 10viral particles) to be further assessed in a phase 2 clinical trial.
The Ad5 vectored COVID-19 vaccine was immunogenic, inducing humoral and T-cell responses rapidly in most participants. Onset of detectable immune responses was rapid, with T-cell responses peaking at day 14 after vaccination and antibodies peaking at day 28. The antibody response to the vaccine in the high dose group was slightly greater than that in the middle dose and low dose groups. A single dose of Ad5 vectored COVID-19 vaccine was able to elicit a four-fold increase in binding antibodies to RBD in 94–100% of participants, and a four-fold increase to live virus in 50–75% of participants. Despite differences in magnitudes of the antibodies measured through different methods, there was a strong positive correlation between binding antibodies and neutralising antibody titres to the live virus. High proportions of participants with positive T-cell responses were noted across the all dose groups post-vaccination. The activation of both CD4+ T cells and CD8+ T cells was observed in vaccine recipients, particularly for antigen-specific CD4+ T cells and CD8+ T cells. However, both the specific antibody response and T-cell response induced by vaccination were partly diminished by the presence of high pre-existing anti-Ad5 immunity.
+ and CD8+ T-cell responses played an essential role in immunity. 17 Zhao J
Perlman S T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. , 18 Channappanavar R
Perlman S Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. 19 Wang X
et al. Neutralizing antibodies responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. , 20 Wu F
et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. Currently, correlates of protection for a vaccine against COVID-19 are unknown, and the roles of the specific antibodies or T cells in building effective protection are not yet defined. Therefore, we are unable to predict the protection of the Ad5 vectored COVID-19 vaccine on the basis of the vaccine-elicited immune responses in this study. However, previous studies investigating SARS and Middle East respiratory syndrome (MERS) found that the increases in specific antibodies were temporary, and declined quickly in patients after recovery, whereas the specific CD4and CD8T-cell responses played an essential role in immunity.A similar rapid decline of the specific antibody amounts in patients with COVID-19 after recovery was also noted,suggesting that both specific cellular and humoral immunity are potentially important for a successful COVID-19 vaccine. Here, we only report the data within 28 days after the vaccination, but we are going to follow up the vaccine recipients for at least 6 months, so more data will be obtained.
et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. This study was done in Wuhan, Hubei province, which was the centre of the COVID-19 epidemic in China.People living in the city of Wuhan had a much higher risk of SARS-CoV-2 infection compared with those living in other cities outside of Hubei province, even though when we initiated this trial, the city had already begun lockdown and implemented mandatory home isolation for residents. Therefore, we did serological screening, nucleic acid testing, and chest CT to exclude participants who had been previously exposed to SARS-CoV-2 during recruitment. In addition, we arranged for all participants in our study to stay in a designated hotel for 14 days post-vaccination. This arrangement facilitated the observation of adverse events after the immunisation of the participants, and reduced the risk of SARS-CoV-2 exposure during the following 2 weeks. These measures allowed the study to be done successfully without interference by the circulation of SARS-CoV-2, which is especially important in the absence of a placebo control.
Murdoch DR Clinical course and mortality risk of severe COVID-19. , 22 Ye G
et al. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. 23 Lurie N
Halton J Developing Covid-19 vaccines at pandemic speed. 24 Cao X COVID-19: immunopathology and its implications for therapy. 25 Venkatraman N
et al. Safety and immunogenicity of a heterologous prime-boost Ebola virus vaccine regimen in healthy adults in the United Kingdom and Senegal. , 26 Dolzhikova IV
et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: an open phase I/II trial in healthy adults in Russia. , 27 Shukarev G
Douoguih M A two-dose heterologous prime-boost vaccine regimen eliciting sustained immune responses to Ebola Zaire could support a preventive strategy for future outbreaks. Interpretation of the results of this study is limited by the small size of the cohort, the short duration of follow-up, and the absence of a randomised control group. As it was a first-in-human study of the Ad5 vectored COVID-19 vaccine, it was not designed to measure the vaccine efficacy. However, in preclinical studies, seven out of eight ferrets were protected from having detectable virus copies when challenged by SARS-CoV-2 through nasal dripping 21 days after immunisation with the vaccine, whereas only one out of eight ferrets in the control group was negative for virus copies (Wei C, unpublished). We aimed to evaluate the safety and tolerability of the candidate vaccine in healthy adults, with no interference by underlying diseases or medicines. However, results of our study indicated that older age could have a negative effect on the vaccine-elicited responses to SARS-CoV-2. In this trial, no participants were older than 60 years and only 16% of the participants were older than 50 years, providing limited information on the capability of generating a potent cellular and humoral response in the older population. Since age has also been identified as an independent risk factor for severe disease associated with SARS-CoV-2 infection,and there is a possibility that an even lower immune response might be found in the older population, we are going to include participants who are older than 60 years in the phase 2 study considering this population as an important target population for a COVID-19 vaccine. Additionally, experience with vaccine candidates for SARS and MERS have raised concerns about the antibody-dependent enhancement in participants who are infected with a circulating SARS-CoV-2 post-vaccination.However, this study was not statistically powered to measure any safety outcome, especially for the concerns around immunopathology and antibody-dependent enhancement events associated with the full-length spike glycoprotein vaccine antigen.Our study found that the pre-existing Ad5 immunity could slow down the rapid immune responses to SARS-CoV-2 and also lower the peak of the responses, particularly for humoral immunity. The high pre-existing Ad5 immunity might also have a negative effect on the persistence of the vaccine-elicited immune responses. In previous studies, heterologous prime-boost combinations or homologous prime-boost regimens with Ad5 vectored vaccines were shown to be able to induce more strong and durable immunogenic responses in populations with high pre-existing Ad5 immunity.Nevertheless, limited information is available for the effects of multiple doses of the candidate Ad5 vectored COVID-19 vaccine in humans, which warrants further investigation.
Halton J Developing Covid-19 vaccines at pandemic speed. 28 Richardson S
et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. 29 Yong CY
Tan WS Recent advances in the vaccine development against Middle East respiratory syndrome-coronavirus. 30 Duan J
et al. A human SARS-CoV neutralizing antibody against epitope on S2 protein. , 31 Wang Q
et al. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. 9 WHO
DRAFT landscape of COVID-19 candidate vaccines–30 April 2020. 9 WHO
DRAFT landscape of COVID-19 candidate vaccines–30 April 2020. + T cells. 32 Gray GE
et al. Recombinant adenovirus type 5 HIV gag/pol/nef vaccine in South Africa: unblinded, long-term follow-up of the phase 2b HVTN 503/Phambili study. Over the past decade, the vaccine industry and clinical research centres have been asked to provide urgent responses to epidemics of emerging infectious diseases, such as H1N1 influenza, Ebola virus, Zika, MERS, and now SARS-CoV-2.The risk of COVID-19 caused by SARS-CoV-2 is ongoing, making the need for effective vaccines even more urgent.We started the development of this candidate vaccine in January, 2020, when SARS-CoV-2 was first isolated and sequenced. The full-length spike glycoprotein was selected as the vaccine antigen, mainly on the basis of previous experience with SARS and MERS vaccines. Previous findings suggested that those vaccines expressing full-length spike glycoprotein can induce good immune responses and protective efficacy.Although the RBD comprises the critical neutralising domains for the coronaviruses, the neutralising epitopes located outside the RBD were also identified.The full-length spike was chosen in most of the viral vectored, mRNA, or DNA COVID-19 vaccines in development.The Ad5 vector vaccine platform is highly efficient and well established as a vaccine antigen delivery system. In addition to our candidate Ad5 vectored COVID-19 vaccine, there are several other Ad5-based vaccines against COVID-19 listed in the WHO draft landscape of COVID-19 candidate vaccines, including Ad5 S (GREVAXTM platform) in the USA, and Oral Ad5 S (Stabilitech Biopharma) in the UK.However, aside from pre-existing anti-Ad5 immunity, there is a concern about the increased risk of HIV-1 acquisition associated with Ad5 activated CD4T cells.Although the association between HIV-1 acquisition risk and Ad5 vectored vaccine is controversial and its mechanism is unclear, the potential risks should be taken into account in studies with this viral vector delivery platform. We plan to monitor the participants in our upcoming phase 2 and phase 3 studies to assess the indication for any such acquisition.
pizzorelli on May 23rd, 2020 at 12:52 UTC »
Who is the manufacturer of this vaccine?
HilariouslySkeptical on May 23rd, 2020 at 11:55 UTC »
Cautiously optimistic.
Chacattack on May 23rd, 2020 at 11:06 UTC »
Can I ask a question, for someone who has more knowledge about scientific studies than me? They write that they gave a low dose (n=36), medium dose (n=36) and a high dose (n=36). Is there any reason they didn't use a control group that were given an injection without any active ingredients? Second question - There was no mention of the person administering the injection not being aware of which dose they were giving the recipient. I thought this was common practice to prevent any undue influence on the results. Would they have done this as standard (which is why it's not explicitly mentioned)? Or did they not do this and is there a valid reason for that?
Edit: spelling