Δ9-THC exposure in the rat does not affect maternal weight or food intake
Pregnant rat dams received either daily doses of vehicle or Δ9-THC (3 mg/kg i.p.) from embryonic day 6.5 (E6.5) through E22. To evaluate maternal outcomes, gestational length, average food intake, pregnancy weight gain, litter size and live birth index were measured. In agreement with previous rodent studies12,19,46,47, daily administration of Δ9-THC to pregnant dams had no effect on maternal weight gain during pregnancy, or maternal food intake (Table 1). In addition, Δ9-THC (3 mg/kg i.p.) did not alter gestational length, litter size or live birth index similar to previous studies with maternal Δ9-THC exposure (Table 1)46,47.
Table 1 Maternal and neonatal outcome measurements. Full size table
Maternal Δ9-THC exposure results in symmetrical IUGR
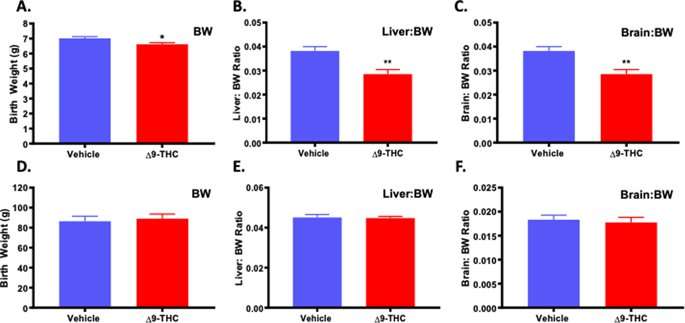
To determine the effect of Δ9-THC exposure on neonatal outcome, assessments included pup weight, and organ to body weight ratio (hallmarks of growth restriction) along with survival to post-natal day (PND)4. A small for gestational age (SGA) birth is <10th percentile for gestational age, or more than 2 standard deviations below the mean, while Intrauterine Growth Restriction (IUGR) refers to a reduction in expected fetal growth48, thus, not all IUGR births are SGA48,49,50,51. Further, growth restriction can be asymmetric, meaning there is first a restriction of weight, followed by length with a “head sparing” effect51. This is the most common form of IUGR, and is seen with pre-eclampsia, hypertension and uterine pathologies51,52. Symmetric growth restriction affects all growth parameters and affects the fetus in a uniform manner and can result in permanent neurological consequences. Symmetric growth restriction is more often the result of genetic causes, intrauterine infections and maternal alcohol use51,52. At birth, the pups from Δ9-THC exposed pregnancies were growth restricted and weighed significantly less than the vehicle control pups (p = 0.001; Table 1). Moreover, in the Δ9-THC group, 2 out 8 dams had one pup that was small for gestational age (SGA) (<2 STD of mean body weight) while the vehicle group had none. Building on a previous study that identified that exposure to cannabis smoke lead to impaired fetal organ development53, PND1 neonates were sacrificed to examine organ-to-body weight ratios and Δ9-THC pups exhibited a ~25% decrease in both liver-to-bodyweight ratio and brain-to-bodyweight ratio (p < 0.01), indicating symmetrical IUGR (Fig. 1). However, the reduced fetal size of the pups from the Δ9-THC exposed pregnancies did not affect survival to PND4 (Table 1).
Figure 1 Exposure to 3 mg/kg Δ9-THC during gestation leads to symmetrical fetal growth restriction followed by postnatal catch-up growth. (A) birth weight, (B) liver:body weight ratio at birth, and (C) brain: body weight ratio at birth. (D) body weight at 3 weeks, (E) liver:body weight ratio at 3 weeks, and (F) brain: body weight ratio at 3 week. Mean ± SEM, average weight/litter, N = 8 dams/group, Significance; Student’s t-test (*P < 0.05, **P < 0.001). Full size image
Pups from Δ9-THC exposed pregnancies experience post-natal catch-up growth
As we have previously demonstrated that post-natal catch up growth in the rat exacerbates the incidence of MetS54,55,56, the pups were evaluated to see if they might be at increased risk. At PND21, pups from the Δ9-THC exposed pregnancies had exhibited catch-up growth with no significant difference in weight, liver to weight ratio or brain to weight ratio (Fig. 1).
Placental weights increased at E19.5, with reduced fetal to placental weight ratio
To explore whether changes in placental structure and composition may underlie the fetal growth restriction observed, a cohort of vehicle and Δ9-THC exposed pregnant dams were sacrificed at E19.5 and fetal and placental weights were evaluated. Similar to PND1, the litter size at E19.5 was not altered between vehicle and Δ9-THC exposed dams (Table 2), nor was the number of reabsorptions significantly different (Table 2). The fetal weights in both treatment groups were the same, suggesting that the overall growth restriction identified at birth, takes place after E19.5. The fetal to placental weight ratio can be used as a measure of placental efficiency57,58. The placentae from Δ9-THC exposed pregnancies were significantly larger than the placentae from vehicle control exposed dams (p < 0.001), causing the fetal to placental weight ratio to be reduced (p < 0.05, Table 2).
Table 2 Fetal and placental outcome measurements at E19.5. Full size table
Structure and composition of trophoblast cells of the junctional zone were unaltered in placentae from Δ9-THC exposed pregnancies
To determine whether structural changes in the placenta contributed to the increase in placental weights, histological assessment of the placental layers was performed. There was no change in the relative size of the junctional zone (Fig. 2A) between the vehicle treated controls and Δ9-THC exposed groups. Furthermore, histological analysis revealed no difference in the junctional zone composition of glycogen trophoblast (Gly-T) or spongiotrophoblast (Sp-T) populations (Fig. 2B) that make up this layer. It is worth noting that while 3 mg/kg Δ9-THC i.p. in the rat did not alter these populations, in mice, pregnancy exposure of 5 mg/kg Δ9-THC i.p. reported junctional zone disorganization with reduced glycogen trophoblast and the spongiotrophoblast populations12. It is possible that the higher dose of Δ9-THC may be more toxic to the junctional zone trophoblast and that 3 mg/kg allows for junctional zone specific trophoblast survival, though it must be considered that it could be a difference between species.
Figure 2 Exposure to 3 mg/kg Δ9-THC during gestation has no measurable effect on junctional zone size or composition at E19.5. (A) Percentage of junctional zone area of total placenta. (B) Analysis of the glycogen trophoblast (Gly-T) and spongiotrophoblast (Sp-T) complement of the junctional zone. (C) Percentage of PAS staining in Gly-T in junctional zone. For junctional zone, 6-images/placenta were taken at 10x. Graphs present mean ± SEM. Full size image
Given that Gly-T in the junctional zone store glycogen and storage can be altered in placentae that are functionally abnormal, we examined whether there was a greater accumulation of glycogen or aldohexoses in the placenta, as observed in other models of placental insufficiency59. PAS staining was performed on serial placental sections without and with diastase treatment to assess for the levels of total aldohexoses vs aldohexoses without glycogen, respectively. In the junctional zone of Δ9-THC placentae, diastase treatment confirmed that glycogen accumulated normally in glycogen trophoblast and that there was no difference in total aldohexoses between vehicle and treated placentae (Fig. 2C).
Larger labyrinth layer, with a reduction in EPCAM+ labyrinth progenitors in placentae from Δ9-THC exposed dams
Histological assessment of the placental layers revealed that the relative area of the labyrinth layer was increased in the placentae from Δ9-THC exposed dams (p < 0.05; Fig. 3A). It has been shown in the rat placenta that proliferation is highest in the labyrinth at E10-11 and has dropped to a basal level by E1660. At E19.5, proliferating cells are much less likely to be observed; however, as proliferation can be altered in response to placenta stress61, it was evaluated to see whether, the rate of proliferation, albeit low, was changed. The increased size was neither attributed to an increase in proliferation, as the number of Ki67+ nuclei was not altered (Fig. 3B), nor the number of sinusoidal trophoblast giant cells (S-TGCs) as there was no difference between treatment groups (Fig. 3B). Interestingly, the EPCAM+ trophoblast progenitor cells that give rise to the differentiated trophoblast of the labyrinth layer appeared fewer in the placentae exposed to Δ9-THC, and qPCR assessment, confirmed that Epcam expression was reduced (p = 0.04600) in response to exposure (Fig. 3C). To further investigate this finding and to determine if syncytiotrophoblast, which differentiate from EPCAM+ trophoblast precursors, were affected, we assessed the expression of Gcm1 by qPCR. Interestingly, Gcm1 expression was not altered by Δ9-THC exposure (Supplemental Fig. 1).
Figure 3 Exposure to 3 mg/kg Δ9-THC during gestation leads to increased labyrinth layer area at E19.5 compared to vehicle treatment, however with no associated increases in cell proliferation nor number of S-TGCs. (A) Percentage of labyrinth layer area of total placenta and representative images showing Iso-Lectin B4 staining in labyrinth. (B) Analysis of numbers of Ki67+ nuclei and S-TGC nuclei in the labyrinth layer. (C) Quantification of Epcam mRNA in rat placenta at E19.5 by qPCR (graph) and assessment of EPCAM protein expression by IHC in labyrinth layer. For labyrinth area, 6-images/placenta were taken at 10×, while for Ki67, S-TGC and Epcam assessment, 6-images/placenta were taken at 40×. Graphs present mean ± SEM. Significance; Student’s t-test (*P < 0.05). Scale bars = 500 uM in (A), 150 uM in (C). Full size image
Following gestational Δ9-THC exposure, rat placentae exhibit vascular defects
The labyrinth zone is the site of maternal-fetal exchange and alterations in vascular development are critical and can contribute to fetal growth restriction. To explore whether the fetal growth restriction observed in Δ9-THC pups could be attributed to placental insufficiency, the fetal capillary network and maternal blood sinusoids within the labyrinth layer (herein referred to as fetal and maternal blood spaces, respectively), were assessed62,63,64,65. The assessment included: area of blood spaces as a percentage of the field of view; maternal to fetal blood space ratio and the perimeter to area ratio, all indicators of surface available for nutrient exchange. The maternal blood space area was increased (p < 0.0001) in response to Δ9-THC exposure, with the perimeter/area ratio of the maternal blood spaces reduced (p < 0.05; Fig. 4A). Furthermore, the fetal blood space area was reduced (p < 0.001) in the placentae from Δ9-THC exposed dams, with an increased fetal perimeter to area ratio (p < 0.05; Fig. 4B,D). Collectively, the maternal/fetal blood space ratio was increased in the labyrinth zone of Δ9-THC placentae (p < 0.0001; Fig. 4C).
Figure 4 Exposure to 3 mg/kg Δ9-THC during gestation leads to increased maternal blood space to fetal blood space ratio in the labyrinth zone at E19.5 compared to vehicle treatment. (A) Percentage of maternal blood area and maternal blood space perimeter/area ratio in labyrinth zone. (B) Percentage of fetal blood area and fetal blood space perimeter/area ratio in labyrinth zone. (C) Maternal blood space to fetal blood space ratio in labyrinth zone. (D) Representative images of fetal blood spaces identified by Iso-Lectin B4 staining. 6-images/placenta were taken at 40×. Graphs present mean ± SEM. Significance; Student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.001 ****P < 0.0001). Scale bar = 100 uM. Full size image
With fetal blood space altered, components that contribute to blood space formation, structure, integrity and function were further evaluated. It is well established that pericytes associate with endothelial cells and wrap around the walls of the fetal capillaries in the placenta. In addition to providing structural support, they, along with trophoblast and fetal endothelial cells contribute to the extracellular matrix (ECM) of the placenta and are suggested to play a role in vascular remodeling and maturation66. Immunohistochemistry (IHC) revealed that α-SMA+ labyrinth pericyte area was increased (p < 0.05; Fig. 5A) in the placentae from Δ9-THC exposed dams, when compared with vehicle treated controls. Collagen IV, an ECM component, was increased (p < 0.05; Fig. 5B), while laminin, another ECM component, was not significantly altered (Fig. 5C). Notably, there was increased PAS staining in the labyrinth zone of Δ9-THC placentae (p < 0.01) but given that diastase treatment did not affect this PAS staining, this is not attributed to an increase in glycogen storage (Fig. 5D). Likely, the increased PAS staining was reflective of changes to components of the ECM/basement membrane.
Figure 5 Exposure to 3 mg/kg Δ9-THC during gestation leads to increased pericyte and collagen area in the labyrinth zone at E19.5 compared to vehicle treatment. (A) Percentage of αSMA+ pericytes area and representative IHC for aSMA staining in labyrinth zone. (B) Percentage of collagen IV staining and representative IHC for collagen IV staining in the labyrinth. (C) Percentage of laminin staining and representative IHC for laminin staining in the labyrinth. (D) Percentage of PAS staining and representative PAS images in the labyrinth. 6-images/placenta were taken at 40x, graphs represent mean ± SEM, Significance; Student’s t-test (*P < 0.05, ***P < 0.001). Scale bars in (A–C) = 120uM; in (D) = 30 uM. Full size image
Δ9-THC exposure results in reduced GLUT1 and GR in vivo
The placenta adapts its nutrient transport system in response to the maternal environment. Glucose is the primary nutrient required for the growth of both the placenta and the fetus. The fetus is dependent on glucose uptake from maternal circulation across the interhemal membrane of the placenta by members of the facilitated glucose transporter family (GLUTs). GLUT1 is the primary glucose transporter and is highly expressed in the placenta throughout both rodent and human pregnancy45,67,68,69. As the primary glucose transporter, GLUT1 is regularly evaluated in several models of IUGR64,68,70,71,72. Thus, upon observation of fetal growth restriction and altered placental blood spaces in placentae from Δ9-THC pregnancies, the expression of GLUT1 was evaluated. GLUT1 was not altered in the junctional zone of placentae from Δ9-THC exposed dams; however, it was significantly reduced in the labyrinth layer (p < 0.05; Fig. 6A,B). A transgenic glucocorticoid receptor deficient mouse study has previously demonstrated that reduced placental GR expression is accompanied by a decrease in GLUT1, resulting in growth restricted pups73. As Δ9-THC has been shown to interact with glucocorticoid receptor (GR)74,75, and GR-signaling mediates GLUT1 expression73,76, GR expression was evaluated in both placental zones. Interestingly, GR positive nuclei were reduced in the labyrinth layer of Δ9-THC placentae (p < 0.05), but not the junctional zone (Fig. 6C,D).
Figure 6 Exposure to 3 mg/kg Δ9-THC during gestation leads to decreased GLUT1 and GR exclusively in the labyrinth zone at E19.5 compared to vehicle treatment. (A) Percentage of GLUT1 area and representative IHC for GLUT1 in the labyrinth layer of placentae from vehicle and Δ9-THC exposed dams. (B) Percentage of GLUT1 area junctional zone. (C) Percentage of GR area and representative IHC for GR in the labyrinth layer of placentae from vehicle and Δ9-THC exposed dams. (D) Percentage of GR area in the junctional zone. For labyrinth layer, 6-images/placenta were taken at 40x, while for junction zone 6-images/placenta were taken at 10x. Graphs present mean ± SEM, Significance; Student’s t-test (*P < 0.05). Scale bars = 30 uM. Arrows indicate positive staining for GR in (C). Full size image
Δ9-THC exposure in human trophoblast results in reduced GLUT1 and GR, in vitro
It is of paramount importance, when using animal models to study human pregnancy related pathology, to evaluate whether observations are of relevance to the human. BeWo cells were derived from a human choriocarcinoma and are well published as a model of human villous trophoblast77,78,79, and have been used as an in vitro model to examine the effects of Δ9-THC on placental function12,80,81,82. Thus BeWo cells were cultured with and without 15 µM Δ9-THC or its inactive metabolite, 11-COOH-THC, to explore the direct effects of Δ9-THC on GLUT1 expression. 15 µM was chosen as the experimental dose based on studies, which determined equivalent doses to those found in the serum of cannabis users and did not affect cellular viability in BeWo cells12,80,82,83. Treatment with Δ9-THC led to decreases in the steady-state mRNA levels of GLUT1 and GR (p < 0.05), while the metabolite (11-COOH-THC) at an equimolar concentration, had no effect (Fig. 7A,B).
timothypjr on January 18th, 2020 at 00:28 UTC »
As an avid pot smoker, I can’t imagine smoking (or drinking, or smoking tobacco, or drugging of any kind) while pregnant.
prof_the_doom on January 17th, 2020 at 23:28 UTC »
This result is no different than anyone should have expected, certainly I didn't.
You're not supposed to drink alcohol, consume more 1-2 cups of coffee worth of caffeine a day, no nicotine, and even a lot of prescription and OTC drugs aren't recommended while pregnant.
I mean seriously, look at this list of things you shouldn't do while pregnant.
civver3 on January 17th, 2020 at 21:51 UTC »
Would the experts in the comments criticizing the use of a rat model care to elaborate which specific physiological differences completely invalidate the results obtained in the study?