For the first time, scientists (Minerbi et al., 2019) have identified a correlation between specific gut microbiome assemblage and a disease (fibromyalgia), which is characterized by widespread , sleep impairments, and fatigue. These findings by a Montreal-based team of researchers, "Altered Microbiome Composition in Individuals with Fibromyalgia," were published online ahead of print this month in the journal Pain.
The interdisciplinary team of scientists involved in this study has affiliations with McGill University, Université de Montréal, and the Research Institute of the McGill University Health Centre (MUHC).
The Canadian researchers also discovered that the severity of someone's fibromyalgia symptoms were directly correlated with an increased presence of certain gut bacteria and a conspicuous absence of other gut microbiome species. According to the researchers, this is something that hasn't been observed or reported until now.
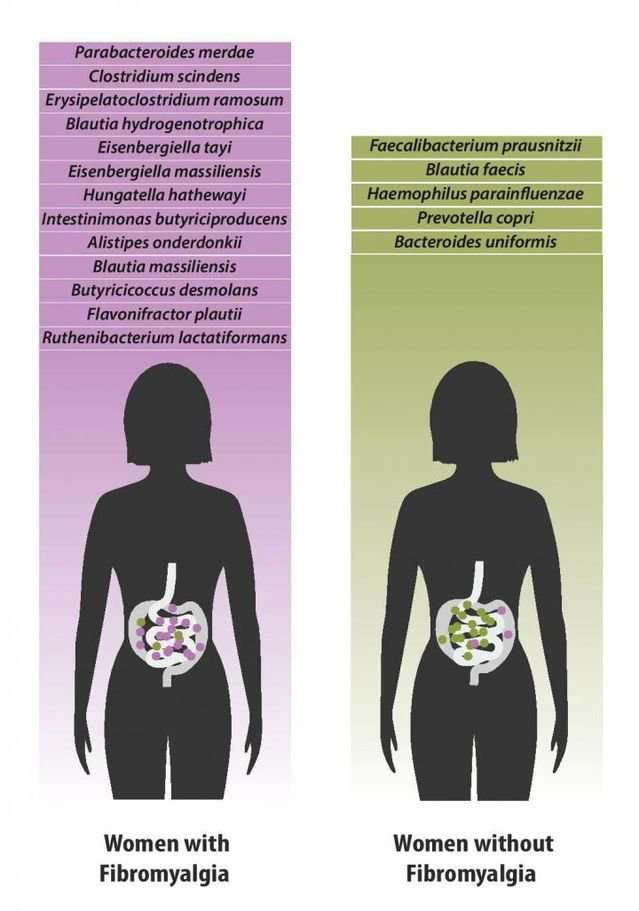
As you can see in the diagram (below) by first author Amir Minerbi, a side-by-side comparison of women with and without fibromyalgia revealed over a dozen different species of gut bacteria in the gastrointestinal tract of study participants with fibromyalgia in comparison to a healthy control group without the disease.
Nota bene: Identifying a correlation between fibromyalgia and specific gut microbiome species does not mean that these microbiota cause the disease. These initial findings are not causal, but instead, offer insights into a potential microbiome-based marker for the disease. As the news release clearly states: "At this point, it's not clear whether the changes in gut bacteria seen in patients with fibromyalgia are simply markers of the disease or whether they play a role in causing it." Future research will drill down on whether specific gut microbiome plays a causal role in the development of various symptoms (e.g., chronic pain) associated with fibromyalgia.
These findings could lead to a breakthrough in diagnosing fibromyalgia. Because it can take as long as four-to-five years for someone with fibromyalgia to receive a final diagnosis, if scientists can pinpoint a specific assemblage of gut microbiome that is universally correlated with fibromyalgia, it could lead to a speedier method of diagnosing this debilitating disease.
How Was This Pioneering Research on Gut Microbiome and Fibromyalgia Conducted?
For this relatively small study, the cohort involved a total of 156 individuals from the Montreal area, 77 of whom had been diagnosed with fibromyalgia. A cohort of 79 individuals without fibromyalgia were used as a control group. Many of the study participants with and without fibromyalgia lived in the same household or were biologically related.
"We used a range of techniques, including , to confirm that the changes we saw in the microbiomes of fibromyalgia patients were not caused by factors such as , , physical activity, age, and so on, which are known to affect the microbiome," Amir Minerbi, from the MUHC Alan Edwards Pain Management Unit, said in a statement.
Because this pioneering research is still in its infancy, follow-up studies are needed to learn more about the possible link between fibromyalgia and unique gut microbiome composition. The next step for Minerbi and colleagues is to see if these soon-to-be-published findings can be replicated using a cohort of human participants from another part of the world. Also, future animal studies could be designed to unearth a possible causal link between the colonization of specific gut bacteria and the development of symptoms associated with fibromyalgia.
zulan on June 24th, 2019 at 13:22 UTC »
Other than fecal transfer, has any research been done on how to balance gut bugs?
woodmeneer on June 24th, 2019 at 11:32 UTC »
I’ve heard that faecal transplants can have positive effects on patients with Crohn’s disease and probably other inflammatory bowel diseases. Researchers could try this if a causal relationship seems likely.
mvea on June 24th, 2019 at 11:00 UTC »
The title of the post is a copy and paste from the first and third paragraphs of the linked academic press release here:
Journal Reference:
Altered microbiome composition in individuals with fibromyalgia
Minerbi, Amir1; Gonzalez, Emmanuel2,3; Brereton, Nicholas J.B.4; Anjarkouchian, Abraham5; Dewar, Ken3,6; Fitzcharles, Mary-Ann1,7; Chevalier, Stéphanie5,8,9; Shir, Yoram1
PAIN: June 18, 2019
Link: https://journals.lww.com/pain/Abstract/publishahead/Altered_microbiome_composition_in_individuals_with.98647.aspx
doi: 10.1097/j.pain.0000000000001640
Abstract
Fibromyalgia (FM) is a prevalent syndrome, characterised by chronic widespread pain, fatigue and impaired sleep, that is challenging to diagnose and difficult to treat. The microbiomes of 77 women with FM and that of 79 control participants were compared using 16S rRNA gene amplification and whole genome sequencing. When comparing FM patients to unrelated controls using differential abundance analysis, significant differences were revealed in several bacterial taxa. Variance in the composition of the microbiomes was explained by FM-related variables more than by any other innate or environmental variable and correlated with clinical indices of FM. In line with observed alteration in butyrate metabolising species, targeted serum metabolite analysis verified differences in the serum levels of butyrate and propionate in FM patients. Using machine learning algorithms, the microbiome composition alone allowed for the classification of patients and controls (ROC AUC 87.8%). To the best of our knowledge, this is the first demonstration of gut microbiome alteration in non-visceral pain. This observation paves the way for further studies, elucidating the pathophysiology of FM, developing diagnostic aids and possibly allowing for new treatment modalities to be explored.