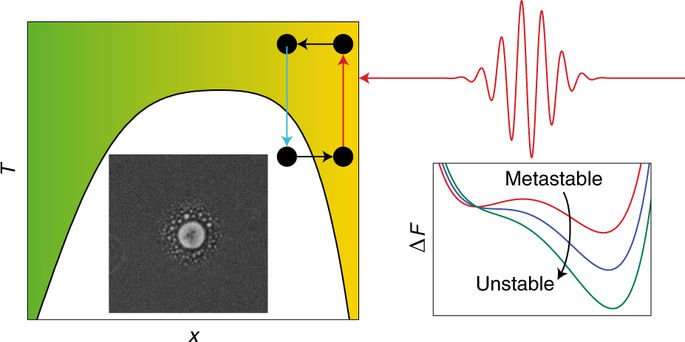
Control over the nucleation of new phases is highly desirable but elusive. Even though there is a long history of crystallization engineering by varying physicochemical parameters, controlling which polymorph crystallizes or whether a molecule crystallizes or forms an amorphous precipitate is still a poorly understood practice. Although there are now numerous examples of control using laser-induced nucleation, the absence of physical understanding is preventing progress. Here we show that the proximity of a liquid–liquid critical point or the corresponding binodal line can be used by a laser-tweezing potential to induce concentration gradients. A simple theoretical model shows that the stored electromagnetic energy of the laser beam produces a free-energy potential that forces phase separation or triggers the nucleation of a new phase. Experiments in a liquid mixture using a low-power laser diode confirm the effect. Phase separation and nucleation using a laser-tweezing potential explains the physics behind non-photochemical laser-induced nucleation and suggests new ways of manipulating matter.
Control over phase separation and nucleation using a laser-tweezing potential
krankie on March 6th, 2018 at 14:34 UTC »
If this process could separate liquids that do not chemically react such as the example of mixing milk and tea given in the title, how would the process appropriately proportion the ratios of the independent liquids upon separation? For example, tea is water and plant material, but milk is similar since it's mostly water combined with animal proteins, enzymes, hormones and other things. But since water is in both liquids, how could it be separated to the original form?
issius on March 6th, 2018 at 13:36 UTC »
So, theoretically this could be used to keep drug costs down, right? One of the issues is that more stable (but ineffective) polymorphs can crystallize over time in the production facilities, rendering it useless.
If we could physically remove those nucleation sites from the synthesis, then you could have a stable production for longer/indefinite periods of time.
jg00de on March 6th, 2018 at 13:24 UTC »
Are there any calculations on how much energy this uses? Trying to rally against thermodynamics at such a molecule to molecule level probably costs alot? Will read paper when I'm at work and no paywalls